Advanced Ultrasound in Diagnosis and Therapy ›› 2024, Vol. 8 ›› Issue (4): 242-249.doi: 10.37015/AUDT.2024.240048
• Original Research • Previous Articles Next Articles
Mohammed Amra,1, Tahmasebi Aylina,1, Kim Soojib,c, Alnoury Mostafaa, E. Wessner Corinnea, Siu Xiao Taniaa, W. Gould Sharona,c, A. May Laurenb,c, Kecskemethy Heidic, T. Saul Davidc, R. Eisenbrey Johna,*( )
)
Received:2024-09-15
Accepted:2024-10-24
Online:2024-12-30
Published:2024-11-12
Contact:
R. Eisenbrey John,
E-mail:John.Eisenbrey@jefferson.edu
About author:First author contact:1 Amr Mohammed and Aylin Tahmasebi contributed equally to this study.
Mohammed Amr, Tahmasebi Aylin, Kim Sooji, Alnoury Mostafa, E. Wessner Corinne, Siu Xiao Tania, W. Gould Sharon, A. May Lauren, Kecskemethy Heidi, T. Saul David, R. Eisenbrey John. Evaluation of Liver Fibrosis on Grayscale Ultrasound in a Pediatric Population Using a Cloud-based Transfer Learning Artificial Intelligence Platform. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(4): 242-249.
Table 1
Baseline characteristics of patients and disease prevalence in the study sample"
| Patient demographics | Results |
|---|---|
| Total number of patients | 190 |
| Males | 95 |
| Females | 95 |
| Mean age | 9.3 ± 6.5 years |
| Disease distribution | Percentage (number) |
| Hepatitis | 27% (51) |
| Abnormal liver function tests | 11.5% (22) |
| MASLD/fatty liver | 20% (38) |
| Biliary conditions* | 14.7% (28) |
| Congenital/genetic conditions** | 11.1% (21) |
| Hematologic conditions*** | 1.5% (3) |
| Miscellaneous | 14% (27) |
Figure 3
(A) Shows sample ultrasound images from a biopsy-confirmed liver fibrosis case that all three radiologists misclassified as normal in all 10 images acquired, whereas AutoML accurately identified fibrosis in all 10 images; (B) Presents sample ultrasound images from a case without liver fibrosis that was correctly identified by all three radiologists in all 10 images, while AutoML consistently misclassified fibrosis in all 10 images."
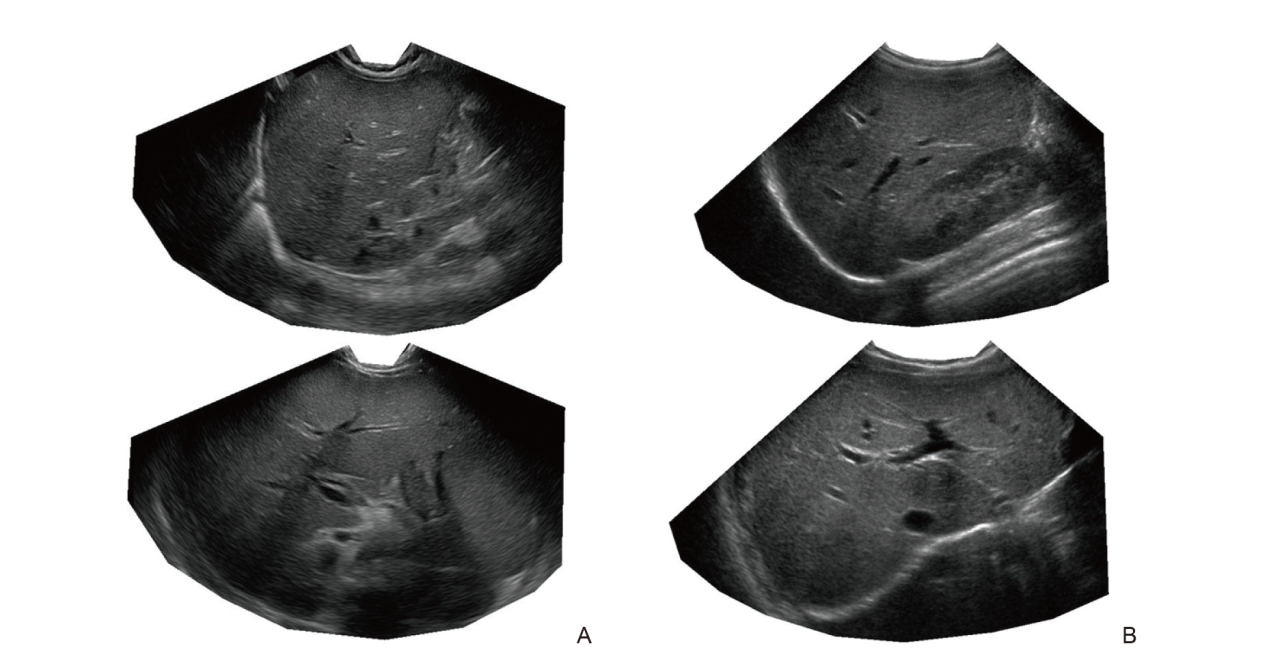
Table 3
Comparative breakdown of the diagnostic performance of the readers vs AutoML"
| Item | R1 | R2 | R3 | AutoML |
|---|---|---|---|---|
| Sensitivity [95% CI] | 46% [38% - 53%] | 39% [32% - 46%] | 40% [33% - 48%] | 70% [63% - 77%] |
| Specificity [95% CI] | 46% [39% - 54%] | 54% [46% - 61%] | 49% [41% - 56%] | 45% [37% - 52%] |
| Positive predictive value [95% CI] | 46% [41% - 51%] | 46% [40% - 52%] | 44% [38% - 50%] | 56% [52% - 60%] |
| Negative predictive value [95% CI] | 46% [41% - 51%] | 47% [42% - 51%] | 45% [40% - 50%] | 60% [53% - 66%] |
| Accuracy [95% CI] | 46% [41% - 51%] | 46% [41% - 52%] | 45% [39% - 50%] | 57% [52% - 62%] |
| Positive likelihood ratio [95% CI] | 0.86 [0.70 - 1.07] | 0.87 [0.68 - 1.10] | 0.8 [0.64 - 1.01] | 1.28 [1.09 - 1.51] |
| Negative likelihood ratio [95% CI] | 1.15 [0.94 - 1.42] | 1.11 [0.93 - 1.33] | 1.2 [0.99 - 1.45] | 0.65 [0.50 - 0.86] |
Table 4
Radiologists Inter-reader agreement in diagnosing pediatric liver fibrosis based on B-mode US images"
| Item | Observed agreements | Kappa value | SE of Kappa | 95% CI | Agreement |
|---|---|---|---|---|---|
| R1 & R2 | 314/360 (87%) | 0.74 | 0.03 | 0.67 - 0.81 | Substantial |
| R1 & R3 | 317/360 (88%) | 0.76 | 0.03 | 0.69 - 0.82 | Substantial |
| R2 & R3 | 335/360 (93%) | 0.85 | 0.02 | 0.80 - 0.91 | Almost perfect |
| [1] |
Ozdogan E, Arikan C. Liver fibrosis in children: a comprehensive review of mechanisms, diagnosis, and therapy. Clin Exp Pediatr 2022; 66:110-124.
doi: 10.3345/cep.2022.00367 pmid: 36550776 |
| [2] | Pediatric Liver Disease: Biliary Atresia & NAFLD [Internet]. 2022 [cited 2024 Jul 23]. Available from: https://liverfoundation.org/about-your-liver/facts-about-liver-disease/pediatric-liver-disease/ |
| [3] |
Lakshminarayanan B, Davenport M. Biliary atresia: A comprehensive review. J Autoimmun 2016; 73:1-9.
doi: 10.1016/j.jaut.2016.06.005 pmid: 27346637 |
| [4] |
Mărginean CO, Meliţ LE, Ghiga DV, Săsăran MO. The assessment of liver fibrosis in children with obesity on two methods: transient and two dimensional shear wave elastography. Sci Rep 2019; 9:19800.
doi: 10.1038/s41598-019-56358-2 pmid: 31875010 |
| [5] | Hunter AK, Lin HC. Review of clinical guidelines in the diagnosis of pediatric nonalcoholic fatty liver disease. Clin Liver Dis 2021; 18:40-44. |
| [6] | Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014; 20:475-485. |
| [7] |
Ovchinsky N, Moreira RK, Lefkowitch JH, Lavine JE. The liver biopsy in modern clinical practice: A pediatric point-of-view. Adv Anat Pathol 2012; 19:250-262.
doi: 10.1097/PAP.0b013e31825c6a20 pmid: 22692288 |
| [8] | Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH and fibrosis. Mol Metab 2021; 50:101167. |
| [9] |
Nogami A, Yoneda M, Iwaki M, Kobayashi T, Kessoku T, Honda Y, et al. Diagnostic comparison of vibration-controlled transient elastography and MRI techniques in overweight and obese patients with NAFLD. Sci Rep 2022; 12:21925.
doi: 10.1038/s41598-022-25843-6 pmid: 36535977 |
| [10] |
Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol 2009; 50:17-35.
doi: 10.1016/j.jhep.2008.10.016 pmid: 19022517 |
| [11] |
Di Serafino M, Severino R, Gioioso M, Rossi E, Vezzali N, Pelliccia P, et al. Paediatric liver ultrasound: a pictorial essay. J Ultrasound 2020; 23:87-103.
doi: 10.1007/s40477-018-0352-z pmid: 30778891 |
| [12] | Choong CC, Venkatesh SK, Siew EPY. Accuracy of routine clinical ultrasound for staging of liver fibrosis. J Clin Imaging Sci 2012; 2:58. |
| [13] |
Mueller-Abt PR, Frawley KJ, Greer RM, Lewindon PJ. Comparison of ultrasound and biopsy findings in children with cystic fibrosis related liver disease. J Cyst Fibros 2008; 7:215-221.
pmid: 17904429 |
| [14] |
He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med 2019; 25:30-36.
doi: 10.1038/s41591-018-0307-0 pmid: 30617336 |
| [15] | Murillo Pineda MI, Siu Xiao T, Sanabria Herrera EJ, Ayala Aguilar A, Arriaga Escamilla D, Aleman Reyes AM, et al. The prediction and treatment of bleeding esophageal varices in the artificial intelligence era: A review. Cureus 2024; 16:e55786. |
| [16] | Rezazade Mehrizi MH, van Ooijen P, Homan M. Applications of artificial intelligence (AI) in diagnostic radiology: a technography study. Eur Radiol 2021; 31:1805-1811. |
| [17] | Ruan D, Shi Y, Jin L, Yang Q, Yu W, Ren H, et al. An ultrasound image‐based deep multi‐scale texture network for liver fibrosis grading in patients with chronic HBV infection. Liver Int 2021; 41:2440-2454. |
| [18] | Xue LY, Jiang ZY, Fu TT, Wang QM, Zhu YL, Dai M, et al. Transfer learning radiomics based on multimodal ultrasound imaging for staging liver fibrosis. Eur Radiol 2020; 30:2973-2983. |
| [19] | Kvostikov AV, Krylov AS, Kamalov UR. Ultrasound image texture analysis for liver fibrosis stage diagnostics. Program Comput Softw 2015; 41:273-278. |
| [20] | Sriharsha Gummadi MD. Automated machine learning in the sonographic diagnosis of non-alcoholic fatty liver disease. Adv ULTRASOUND Diagn Ther 2020; 4:176-182. |
| [21] | Wang S, Xu J, Tahmasebi A, Daniels K, Liu JB, Curry J, et al. Incorporation of a machine learning algorithm with object detection within the thyroid imaging reporting and data system improves the diagnosis of genetic risk. Front Oncol 2020; 10:591846. |
| [22] | Tahmasebi A, Qu E, Sevrukov A, Liu JB, Wang S, Lyshchik A, et al. Assessment of axillary lymph nodes for metastasis on ultrasound using artificial intelligence. Ultrason Imaging 2021; 43:329-336. |
| [23] | Tahmasebi A, Wang S, Wessner CE, Vu T, Liu JB, Forsberg F, et al. Ultrasound-based machine learning aapproach for detection of nonalcoholic fatty liver disease. J Ultrasound Med 2023; 42:1747-1756. |
| [24] | Machado P, Tahmasebi A, Fallon S, Liu JB, Dogan BE, Needleman L, et al. Characterizing sentinel lymph node status in breast cancer patients using a deep-learning model compared with radiologists’ analysis of grayscale ultrasound and lymphosonography. Ultrasound Q 2024; 40:e00683. |
| [25] | Daniels K, Gummadi S, Zhu Z, Wang S, Patel J, Swendseid B, et al. Machine learning by ultrasonography for genetic risk stratification of thyroid nodules. JAMA Otolaryngol-- Head Neck Surg 2020; 146:36-41. |
| [26] | Faes L, Liu X, Wagner SK, Fu DJ, Balaskas K, Sim DA, et al. A clinician’s guide to artificial intelligence: How to critically appraise machine learning studies. Transl Vis Sci Technol 2020; 9:7. |
| [27] | Liu Y, Chen PHC, Krause J, Peng L. How to read articles that use machine learning: Users’ guides to the medical literature. JAMA 2019; 322:1806-1816. |
| [28] |
Duan YY, Qin J, Qiu WQ, Li SY, Li C, Liu AS, et al. Performance of a generative adversarial network using ultrasound images to stage liver fibrosis and predict cirrhosis based on a deep-learning radiomics nomogram. Clin Radiol 2022; 77:e723-731.
doi: 10.1016/j.crad.2022.06.003 pmid: 35811157 |
| [29] |
Liu Z, Wen H, Zhu Z, Li Q, Liu L, Li T, et al. Diagnosis of significant liver fibrosis in patients with chronic hepatitis B using a deep learning-based data integration network. Hepatol Int 2022; 16:526-536.
doi: 10.1007/s12072-021-10294-4 pmid: 35312969 |
| [30] | Feng X, Chen X, Dong C, Liu Y, Liu Z, Ding R, et al. Multi-scale information with attention integration for classification of liver fibrosis in B-mode US image. Comput Methods Programs Biomed 2022; 215:106598. |
| [31] | Lee JH, Joo I, Kang TW, Paik YH, Sinn DH, Ha SY, et al. Deep learning with ultrasonography: automated classification of liver fibrosis using a deep convolutional neural network. Eur Radiol 2020; 30:1264-1273. |
| [32] | Al-Hasani M, Sultan LR, Sagreiya H, Cary TW, Karmacharya MB, Sehgal CM. Ultrasound radiomics for the detection of early-stage liver fibrosis. Diagnostics 2022; 12:2737. |
| [33] | Cheng HY, Wang HY, Chang WH, Lin SC, Chu CH, Wang TE, et al. Nonalcoholic fatty liver disease: Prevalence, influence on age and sex, and relationship with metabolic syndrome and insulin resistance. Int J Gerontol 2013; 7:194-198. |
| [34] | Heyens LJM, Busschots D, Koek GH, Robaeys G, Francque S. Liver fibrosis in non-alcoholic fatty liver disease: From liver biopsy to non-invasive biomarkers in diagnosis and treatment. Front Med 2021; 8:615978. |
| [35] | Krishnan G, Singh S, Pathania M, Gosavi S, Abhishek S, Parchani A, et al. Artificial intelligence in clinical medicine: catalyzing a sustainable global healthcare paradigm. Front Artif Intell 2023; 6:1227091. |
| [36] | Das A, Connell M, Khetarpal S. Digital image analysis of ultrasound images using machine learning to diagnose pediatric nonalcoholic fatty liver disease. Clin Imaging 2021; 77:62-68. |
| [37] |
Zhou W, Yang Y, Yu C, Liu J, Duan X, Weng Z, et al. Ensembled deep learning model outperforms human experts in diagnosing biliary atresia from sonographic gallbladder images. Nat Commun 2021; 12:1259.
doi: 10.1038/s41467-021-21466-z pmid: 33627641 |
| [38] | Hsu FR, Dai ST, Chou CM, Huang SY. The application of artificial intelligence to support biliary atresia screening by ultrasound images: A study based on deep learning models. PLOS ONE 2022; 17:e0276278. |
| [1] | Shama Shiti, Xie Xinxin, Wu Ruiqi, He Ping, Li Xiaoda, Chen Qingfeng, Liang Xiaolong. Advancements in BaTiO3-Based Ultrasound‐Triggered Piezoelectric Catalysis for Tumor Therapy [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(4): 231-241. |
| [2] | Yuzhou Shen, MD, Lin Jin, MD, Lei Sha, MD, Mengmeng Cao, MD, Desheng Sun, MD, Li Liu, MD, Zhaojun Li, MD. Can Different Expertise Levels of Ultrasound Operators Accurately Screen with Handheld Ultrasound? [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(3): 116-123. |
| [3] | Yuhang Zheng, BS, Jianqiao Zhou, MD. Deep Learning in Ultrasound Localization Microscopy [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(3): 86-92. |
| [4] | Hao Feng, MM, Yaqin Sun, MM, Jingjing Zhang, MM, Jiajia Wang, MM, Shuai Han, MM, Shumin Wang, PhD. Ultrasound Assessment of Effect of Maternal Thyroid Function During Pregnancy on Fetal and Neonatal Bone Development [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(2): 41-48. |
| [5] | Lingyun Jia, MD, PhD, Yuan Li, PhD, Yang Hua, MD, Yumei Liu, MD, Nan Zhang, MD, Mingjie Gao, MD, Ke Zhang, MD, Jingzhi Li, MD, Benchi Chen, BS, Jidong Mi, MS, Nan Zhao, PhD. Evaluation of Atherosclerosis Development by Vascular Duplex Ultrasonography in ApoE-deficient Dogs Fed with a High-fat Diet [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(2): 49-56. |
| [6] | Cong Wei, MD, Hui Zhang, PhD, Tao Ying, MD, Bing Hu, MD, Yini Chen, MD, Hongtao Li, MD, Qiude Zhang, PhD, Mingyue Ding, PhD, Jie Chen, MD, Ming Yuchi, PhD, Yuanyi Zheng, MD. Clinical Application of Ultrasound Tomography in Diagnosis of Musculoskeletal Diseases [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(1): 7-14. |
| [7] | Hui Li, MD, Nan Zheng, MD, Penglin Zou, MD, Chao Jia, MD, Long Liu, MD, Gang Li, MD, Ziqi Wang, MD, Rong Wu, MD, Lianfang Du, MD, Qiusheng Shi, MD. The Role of Ultrasonography in the Diagnosis of Systemic Sarcoidosis: a Case Report and Literature Review [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(1): 32-38. |
| [8] | Yang Qi, MD, Dengsheng Sun, MD, Linyao Wang, MD, Jie Yu, MD, Ping Liang, MD. State-of-the-Art and Development Trend of Interventional Ultrasound in China [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 313-320. |
| [9] | Osama Mahmoud, BS, Ajay Makkena, BS, Corinne E. Wessner, MS, MBA, RDMS, Ji-Bin Liu, MD, John R. Eisenbrey, PhD, Andrej Lyshchik, MD, PhD. Contrast-Enhanced Ultrasound LI-RADS: A Pictorial Review [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 321-332. |
| [10] | Jin Guo, MD, Zhaojun Li, PhD, Yanping Lin, PhD. Semi-supervised Learning for Real-time Segmentation of Ultrasound Video Objects: A Review [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 333-347. |
| [11] | Huihui Chai, MS, Xiaowan Bo, MD, Lehang Guo, MD, Chengzhong Peng, MD. Experience and Enlightenment of Handheld Ultrasound Applications in Multiple Scenarios Based on 5G Technology [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 356-365. |
| [12] | Yalin Wu, PhD, Qiaoli Ge, MM, Linyang Yan, PhD, Desheng Sun, MD. A Non-Invasive Follicular Thyroid Cancer Risk Prediction System Based on Deep Hybrid Multi-feature Fusion Network [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 373-380. |
| [13] | Lanxia Zhang, MM, Qingjing Zeng, MD, Guanghui Guo, MM, Xuqi He, MM, Kai Li, MD. An Epstein-Barr Virus Positive Lymphoepithelioma-Like Cholangiocarcinoma in A Young Woman with Chronic Hepatitis B Treated through Microwave Ablation: A Case Report and Literature Review [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 405-408. |
| [14] | Keyan Li, MD, Ye Peng, MD, Yingying Chen, MD, Zhaoming Zhong, MD, Yulong Ma, MD, Tao Yao, MD, Lihai Zhang, MD, Faqin Lv, MD. Robot-assisted Teleultrasound-guided Hemostasis and Hematoma Catheterization and Drainage for Osteoporosis Pelvic Fracture with Giant Hematoma and Active Bleeding [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 416-419. |
| [15] | Wenwen Chen, BS, Yuji Xie, MD, Zisang Zhang, MD, Ye Zhu, MS, Yiwei Zhang, MD, Shuangshuang Zhu, MD, PhD, Chun Wu, MD, PhD, Ziming Zhang, MD, Xin Yang, PhD, Man wei Liu, MD, PhD, Mingxing Xie, MD, PhD, Li Zhang, MD, PhD. Artificial Intelligence-assisted Medical Imaging in Interventional Management of Valvular Heart Disease [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(3): 217-227. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Share: WeChat
Copyright ©2018 Advanced Ultrasound in Diagnosis and Therapy
|
 Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
