Advanced Ultrasound in Diagnosis and Therapy ›› 2023, Vol. 7 ›› Issue (3): 217-227.doi: 10.37015/AUDT.2023.230030
• Review Articles • Previous Articles Next Articles
Wenwen Chen, BSa,b,c, Yuji Xie, MDa,b,c, Zisang Zhang, MDa,b,c, Ye Zhu, MSa,b,c, Yiwei Zhang, MDa,b,c, Shuangshuang Zhu, MD, PhDa,b,c, Chun Wu, MD, PhDa,b,c, Ziming Zhang, MDa,b,c, Xin Yang, PhDa,b,c, Man wei Liu, MD, PhDa,b,c, Mingxing Xie, MD, PhDa,b,c,*( ), Li Zhang, MD, PhDa,b,c,*(
), Li Zhang, MD, PhDa,b,c,*( )
)
Received:2023-04-08
Revised:2023-04-16
Accepted:2023-07-27
Online:2023-09-30
Published:2023-10-09
Contact:
Mingxing Xie, MD, PhD, Li Zhang, MD, PhD,
E-mail:xiemx@hust.edu.cn;zli429@hust.edu.cn
Wenwen Chen, BS, Yuji Xie, MD, Zisang Zhang, MD, Ye Zhu, MS, Yiwei Zhang, MD, Shuangshuang Zhu, MD, PhD, Chun Wu, MD, PhD, Ziming Zhang, MD, Xin Yang, PhD, Man wei Liu, MD, PhD, Mingxing Xie, MD, PhD, Li Zhang, MD, PhD. Artificial Intelligence-assisted Medical Imaging in Interventional Management of Valvular Heart Disease. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(3): 217-227.
Figure 1
Overview of AI-assisted medical imaging in interventional management of VHD. Current AI-assisted medical imaging in interventional management of VHD mainly focuses on disease diagnosis, pre-operative planning, intra-operative navigation, prognosis analysis and risk stratification. Diseases diagnoses are mainly based on aortic stenosis and mitral regurgitation. Pre-operative planning mainly includes plane positioning, simulating valve implantation, and simulating MC implantation. Intra-operative navigation focuses on image fusion, lesion localization, and three-dimensional model reconstruction."
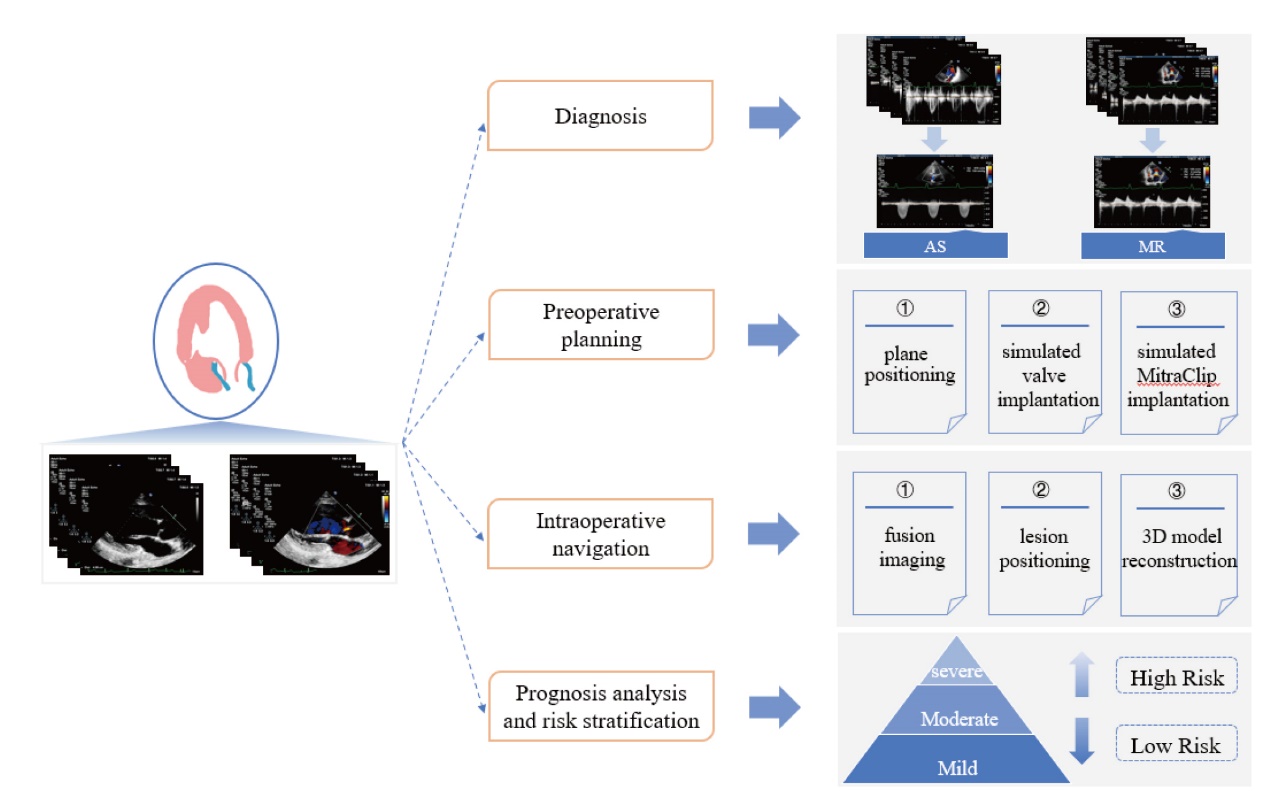
Table 1
AI-assisted medical imaging in diagnosis of VHD"
| Study | VHD | Task | AI methods | Samples | Performance metrics |
|---|---|---|---|---|---|
| Kang, N. G. et al. [ | AS | Diagnosed severe AS | LASSO; RFs; XGBoost | Training: 312 subjects Testing: 96 subjects | AUC: 0.921 (LASSO & XGBoost) |
| Sengupta, P. P. et al. [ | AS | Distinguished AS phenotypes | TDA; ML | 1964 subjects | AUC: 0.988 Accuracy (%): 94.3 Precision (%): 91.3 Recall (%): 95.5 |
| Yang, F. et al. [ | AS | Differential diagnosis with other diseases | ML | Training: 1335 subjects Validation: 311 subjects Testing: 434 subjects | AUC: 0.97 Accuracy (%): 94 Sensitivity (%): 90 Specificity (%): 94 |
| Moghaddasi, H. et al. [ | MR | Detected normal, mild, moderate and severe MR subjects | Textural analysis; SVM; LDA; TM | 5004 images | Accuracy (%): 99.45 (SVM) Accuracy (%): 95.72 (LDA,NN) Accuracy (%): 95 (TM) Sensitivity (%): 99.38 Specificity (%): 99.63 |
| Pimor, A. et al. [ | MR | Distinguished MR phenotypes | DL | 122 subjects | HR: 3.57 (1.72-7.44) |
| Bartko, P. E. et al. [ | MR | Explored the morphological and Functional characteristics of secondary MR | PC; cluster analysis | 383 subjects | HR: 2.18 (clusters3) |
Table 2
AI-assisted medical imaging in pre-operative planning of VHD interventional therapy"
| Study | Operation | Task | Technology | Samples | Performance metrics |
|---|---|---|---|---|---|
| Theriault-Lauzier, P. et al. [ | TAVR | Plane positioning | CNN | 94 subjects | Localization error (mm): 0.9 ± 0.8 (testing) |
| Al, W.A. et al. [ | TAVR | Located important anatomic markers | ML | 71 subjects | Localization error (mm): 2.04 ± 1.11 |
| Rocatello, G. et al. [ | TAVR | Determined the optimal valve size and implantation position | FE | 62 subjects | Accuracy (%): 71 Maximum contact pressure (%): 75 Contact pressure index (%): 71 |
| De Jaegere, P. et al. [ | TAVR | Simulated the TAVR surgical process | FE | 60 subjects | Accuracy (%): 80 Cutoff value (ml/s): 16.0 Sensitivity: 0.72 Specificity: 0.78 |
| Auricchio, F. et al. [ | TAVR | Simulated valve implantation | FE | 2 subjects | Stress state has consistency (between 2 subjects) |
| Astudillo, P. et al. [ | TAVR | Calculated the size of the implanted prosthesis | CNN | Training: 355subjects Testing: 118 subjects | Total analysis time(s): < 1 Device size has consistency (between the manual and automatic selection) |
| Astudillo, P. et al. [ | TMVR | Measured multiple biological parameters | DL | 71 subjects | Total analysis time (s): < 1 |
| Oguz, D. et al. [ | TMVR | Explored the correlation between 3D-TEE parameters and MR reduction | 3D TEE; Mitral Valve Navigator. | 59 subjects | Optimal MR reduction: 68% |
| Guerrero, M. et al. [ | TMVR | Simulated valve implantation | FE | / | Total analysis time: < 3 h |
| Wang, D.D. et al. [ | TMVR | Simulated valve implantation | CAD | 38 subjects | R2: 0.8169 (neo-LVOT surface area) Sensitivity: 100% Specificity: 96.8% |
| Kong, F. et al. [ | TEER | simulated the biomechanics of MC implantation | FE; MC. | 1 subject | Antero-posterior distance: ↓26% Annulus area: ↓19% Valve opening orifice area: ↓48% Regurgitant orifice area: ↓63% Anterior leaflet peak stresses: ↑ 64% Posterior leaflet peak stresses: ↑62% Anterior leaflet peak strains: ↑ 20% Posterior leaflet peak strains: ↑10% |
| Sturla, F. et al. [ | TEER | Simulated the biomechanics of MC implantation | FE; MC. | 3 subjects | Systolic CoA: ↑11-40% Systolic leaflet stresses (Kpa): 100-500 Diastolic leaflet stresses (Kpa): 250 (subject 3) Diastolic orifice area (%): ↓58.9% |
| Caballero, A. et al. [ | TEER | Evaluated the biomechanics of MC implantation | FE; FSI; MC. | 1 subject | Antero-posterior distance: ↓28% Mitral annulus spherecity index: ↓39% Anatomic regurgitant orifice area: ↓52% Anatomic opening orifice area: ↓71% Diastolic anterior leaflet stress: ↑210% Diastolic posterior leaflet stress: ↑145% |
| Mansi, T. et al. [ | TEER | Evaluated the biomechanical impact of mitral valve repair | FE; ML. | 25 subjects | Mean error (mm):1.49 ± 0.62 (ground truth) Mean error (mm):2.75 ± 0.86 (automatic detection) Total analysis time (min): < 14 |
| Dabiri, Y. et al. [ | TEER | Predicted the effect of TEER therapy with MC | DL; XGBoost. | 1267 FE models | MAPE: 54 and 0.310 (DL) MAPE: 0.115 and 0.231 (XGBoost) Total analysis time (s): < 1 |
Table 3
AI-assisted medical imaging in intra-operative navigation of VHD interventional therapy"
| Study | Operation | Task | Technology | Samples | Performance metrics |
|---|---|---|---|---|---|
| Biaggi, P. et al. [ | TAVR | Investigated the efficacy of FS in the perioperative period of TAVR | FI; EN; 3D TEE. | Total: 138subjects FS+: 69subjects FS-: 69subjects | Procedure time (min): 42.1 ± 15.2 (FS+) Procedure time (min): 49.2 ± 20.7 (FS-) Contrast agent use (ml): 34.3 ± 22.0 (FS+) Contrast agent use (ml): 39.0 ± 23.3 (FS-) Fluoroscopy time (min): 11.4 ± 4.7 (FS+) Fluoroscopy time (min): 10.9 ± 5.5 (FS-) Pearson correlation r: 0.63-0.78 Interclass correlation coefficient: 0.95-0.99 |
| Luo, Z. et al. [ | TAVR | Reconstructed aortic valve models and determined the target location for aortic valve prosthesis implantation | MTS; 2D US; 4D CT. | ECG signal | Aortic root segmentation algorithm error (mm): 0.92 ± 0.85 Computational time (ms): 36.13 ± 6.26 Yielding fiducial localization errors (mm): 3.02 ± 0.39 Target registration errors(mm): 3.31 ± 1.55 Deployment distance(mm): 3.23 ± 0.94 Tilting errors (°): 5.85 ± 3.06 |
| Mazomenos, E. B. et al. [ | TAVR | Evaluated surgical skills and verified the role of robot assisted TAVR surgery | FE; k-means clustering; EM. | 12 subjects (novice group: 6 subjects) | The median value of the procedure time (s): 34.9 (stage 1) The median value of the procedure time (s): 111.2 (stage 2) Maximum accuracy (%): 83 (k-means) Maximum accuracy (%): 91 (EM) Average speed (px/s): 22.3 (stage1) Average speed (px/s): 22 (stage2) P = 0.031(conventional equipment vs robotic system ) |
| Prihadi, E. A. et al. [ | TAVR | Quantified aortic ring and root size | 3D TEE; AVN. | 150 subjects | Mean analysis time (min): 4.2 ± 1.0 r≥0.90 (inter- and intra-observer variability) |
| Lang, P. et al. [ | TAVR | Build TAVI's enhanced image guidance system | 3D TEE | / | Mean contour boundary distance error (mm): 1.3 (short-axis views) Mean contour boundary distance error (mm): 2.8 (long-axis views) Mean target registration error (mm): 5.9 |
| Coisne, A. et al. [ | TMVR | Defined the optimal 3D TEE parameters for TMVR | 3D TEE | 57 subjects | AUC: 0.88-0.91 (mitral annular area) AUC: 0.85-0.91 (mitral annular perimeter) |
| Jin, C. N. et al. [ | TMVR | Located MVP | AIUS | 90 subjects | Accuracy (%): 89 (nonexperts) Image analysis time (min): 1.9 ± 0.7 (experts) Image analysis time (min): 5.0 ± 0.5 (nonexperts) |
| Altiok, E. et al. [ | TEER | Evaluated the value of RT 3D TEE | RT 3D TEE; 2D TEE. | 28 subjects | Advantages: 9/11 (RT 3D TEE) |
| Melillo, F. et al. [ | TEER | Explored the TEER treatment effect after MC implantation | FI; MC. | 80 subjects | Fluoroscopy time (min): 37.3 ± 14.6 Procedural time (min): 92.2 ± 36.1 |
| Sündermann, S.H. et al. [ | TEER | Evaluated the feasibility and safety of using MC | EN Software; MC. | 21 subjects | Radiation dose (Gy/cm2): 146.5 ± 123.6 Total procedure time (min): 136.2 ± 50.2 |
| [1] |
Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005-1011.
doi: 10.1016/S0140-6736(06)69208-8 pmid: 16980116 |
| [2] |
Ouyang D, He B, Ghorbani A, Yuan N, Ebinger J, Langlotz CP, et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 2020; 580:252-256.
doi: 10.1038/s41586-020-2145-8 |
| [3] |
Zhang J, Gajjala S, Agrawal P, Tison GH, Hallock LA, Beussink-Nelson L, et al. Fully automated echocardiogram interpretation in clinical practice. Circulation 2018; 138:1623-1635.
doi: 10.1161/CIRCULATIONAHA.118.034338 pmid: 30354459 |
| [4] |
Kang NG, Suh YJ, Han K, Kim YJ, Choi BW. Performance of prediction models for diagnosing severe aortic stenosis based on aortic valve calcium on cardiac computed tomography: incorporation of radiomics and machine learning. Korean J Radiol 2021; 22:334-343.
doi: 10.3348/kjr.2020.0099 pmid: 33236537 |
| [5] |
Sengupta PP, Shrestha S, Kagiyama N, Hamirani Y, Kulkarni H, Yanamala N, et al. A machine-learning framework to identify distinct phenotypes of aortic stenosis severity. JACC Cardiovasc Imaging 2021; 14:1707-1720.
doi: 10.1016/j.jcmg.2021.03.020 pmid: 34023273 |
| [6] |
Yang F, Chen X, Lin X, Chen X, Wang W, Liu B, et al. Automated analysis of Doppler echocardiographic videos as a screening tool for valvular heart diseases. JACC Cardiovascular Imaging 2022; 15:551-563.
doi: 10.1016/j.jcmg.2021.08.015 |
| [7] |
Moghaddasi H, Nourian S. Automatic assessment of mitral regurgitation severity based on extensive textural features on 2D echocardiography videos. Comput Biol Med 2016; 73:47-55.
doi: 10.1016/j.compbiomed.2016.03.026 pmid: 27082766 |
| [8] |
Pimor A, Galli E, Vitel E, Corbineau H, Leclercq C, Bouzille G, et al. Predictors of post-operative cardiovascular events, focused on atrial fibrillation, after valve surgery for primary mitral regurgitation. Eur Heart J Cardiovasc Imaging 2019; 20:177-184.
doi: 10.1093/ehjci/jey049 pmid: 29608669 |
| [9] |
Bartko PE, Heitzinger G, Spinka G, Pavo N, Prausmüller S, Kastl S, et al. Principal morphomic and functional components of secondary mitral regurgitation. JACC Cardiovascular Imaging 2021; 14:2288-2300.
doi: 10.1016/j.jcmg.2021.05.020 |
| [10] |
Theriault-Lauzier P, Alsosaimi H, Mousavi N, Buithieu J, Spaziano M, Martucci G, et al. Recursive multiresolution convolutional neural networks for 3D aortic valve annulus planimetry. Int J Comput Assist Radiol Surg 2020; 15:577-588.
doi: 10.1007/s11548-020-02131-0 pmid: 32130646 |
| [11] |
Al WA, Jung HY, Yun ID, Jang Y, Park HB, Chang HJ. Automatic aortic valve landmark localization in coronary CT angiography using colonial walk. PLoS One 2018; 13:e0200317.
doi: 10.1371/journal.pone.0200317 |
| [12] |
Rocatello G, El Faquir N, de Backer O, Swaans MJ, Latib A, Vicentini L, et al. The impact of size and position of a mechanical expandable transcatheter aortic valve: novel insights through computational modelling and simulation. J Cardiovasc Transl Res 2019; 12:435-446.
doi: 10.1007/s12265-019-09877-2 pmid: 31444672 |
| [13] |
de Jaegere P, De Santis G, Rodriguez-Olivares R, Bosmans J, Bruining N, Dezutter T, et al. Patient-specific computer modeling to predict aortic regurgitation after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2016; 9:508-512.
doi: 10.1016/j.jcin.2016.01.003 |
| [14] |
Auricchio F, Conti M, Morganti S, Reali A. Simulation of transcatheter aortic valve implantation: a patient-specific finite element approach. Comput Methods Biomech Biomed Engin 2014; 17:1347-1357.
doi: 10.1080/10255842.2012.746676 pmid: 23402555 |
| [15] | Astudillo P, Mortier P, Bosmans J, De Backer O, de Jaegere P, De Beule M, et al. Enabling automated device size selection for transcatheter aortic valve implantation. J Interv Cardiol 2019; 2019:3591314. |
| [16] |
Astudillo P, De Beule M, Dambre J, Mortier P. Towards safe and efficient preoperative planning of transcatheter mitral valve interventions. Morphologie 2019; 103:139-147.
doi: S1286-0115(19)30053-0 pmid: 31570309 |
| [17] |
Oguz D, Eleid MF, Dhesi S, Pislaru SV, Mankad SV, Malouf JF, et al. Quantitative three-dimensional echocardiographic correlates of optimal mitral regurgitation reduction during transcatheter mitral valve repair. J Am Soc Echocardiogr 2019; 32:1426-1435.
doi: 10.1016/j.echo.2019.06.014 |
| [18] |
Guerrero M, Urena M, Wang DD, O'Neill W, Feldman T. Reply: patient-specific computer modeling for the planning of transcatheter mitral valve replacement. J Am Coll Cardiol 2018; 72:958.
doi: S0735-1097(18)35280-X pmid: 30115239 |
| [19] |
Wang DD, Eng MH, Greenbaum AB, Myers E, Forbes M, Karabon P, et al. Validating a prediction modeling tool for left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR). Catheter Cardiovasc Interv 2018; 92:379-387.
doi: 10.1002/ccd.v92.2 |
| [20] |
Wang DD, Eng M, Greenbaum A, Myers E, Forbes M, Pantelic M, et al. Predicting LVOT obstruction after TMVR. JACC Cardiovasc Imaging 2016; 9:1349-1352.
doi: S1936-878X(16)30041-9 pmid: 27209112 |
| [21] |
Kong F, Caballero A, McKay R, Sun W. Finite element analysis of MitraClip procedure on a patient-specific model with functional mitral regurgitation. J Biomech 2020; 104:109730.
doi: 10.1016/j.jbiomech.2020.109730 |
| [22] |
Sturla F, Redaelli A, Puppini G, Onorati F, Faggian G, Votta E. Functional and biomechanical effects of the edge-to-edge repair in the setting of mitral regurgitation: consolidated knowledge and novel tools to gain insight into its percutaneous implementation. Cardiovasc Eng Technol 2015; 6:117-140.
doi: 10.1007/s13239-014-0208-4 pmid: 26577231 |
| [23] |
Caballero A, Mao W, McKay R, Hahn RT, Sun W. A comprehensive engineering analysis of left heart dynamics after MitraClip in a functional mitral regurgitation patient. Front Physiol 2020; 11:432.
doi: 10.3389/fphys.2020.00432 pmid: 32457650 |
| [24] |
Mansi T, Voigt I, Georgescu B, Zheng X, Mengue EA, Hackl M, et al. An integrated framework for finite-element modeling of mitral valve biomechanics from medical images: application to MitralClip intervention planning. Med Image Anal 2012; 16:1330-1346.
doi: 10.1016/j.media.2012.05.009 pmid: 22766456 |
| [25] |
Dabiri Y, Mahadevan VS, Guccione JM, Kassab GS. Machine learning used for simulation of MitraClip intervention: A proof-of-concept study. Front Genet 2023; 14:1142446.
doi: 10.3389/fgene.2023.1142446 |
| [26] | Biaggi P, Sager DF, Külling J, Küest S, Wyss C, Hürlimann D, et al. Potential value of fusion imaging and automated three-dimensional heart segmentation during transcatheter aortic valve replacement. J Am Soc Echocardiogr 2020;33:516-517. |
| [27] |
Luo Z, Cai J, Peters TM, Gu L. Intra-operative 2-D ultrasound and dynamic 3-D aortic model registration for magnetic navigation of transcatheter aortic valve implantation. IEEE Trans Med Imaging 2013; 32:2152-2165.
doi: 10.1109/TMI.2013.2275233 |
| [28] | Mazomenos EB, Chang PL, Rippel RA, Rolls A, Hawkes DJ, Bicknell CD, et al. Catheter manipulation analysis for objective performance and technical skills assessment in transcatheter aortic valve implantation. Int J Comput Assist Radiol Surg 2016; 11:1121-1131. |
| [29] |
Prihadi EA, van Rosendael PJ, Vollema EM, Bax JJ, Delgado V, Ajmone Marsan N,. Feasibility, accuracy, and reproducibility of aortic annular and root sizing for transcatheter aortic valve replacement using novel automated three-dimensional echocardiographic software: comparison with multi-detector row computed tomography. J Am Soc Echocardiogr 2018; 31:505-514.
doi: 10.1016/j.echo.2017.10.003 |
| [30] | Lang P., Rajchl M., McLeod A. J., Chu M. & Peters T. Feature identification for image-guided transcatheter aortic valve implantation. Medical Imaging 2012. |
| [31] |
Coisne A, Pontana F, Aghezzaf S, Mouton S, Ridon H, Richardson M, et al. Utility of three-dimensional transesophageal echocardiography for mitral annular sizing in transcatheter mitral valve replacement procedures: a cardiac computed tomographic comparative study. J Am Soc Echocardiogr 2020; 33:1245-1252.
doi: 10.1016/j.echo.2020.04.030 |
| [32] | Jin CN, Salgo IS, Schneider RJ, Kam KK, Chi WK, So CY, et al. Using anatomic intelligence to localize mitral valve prolapse on three-dimensional echocardiography. J Am Soc Echocardiogr 2016; 29:938-945. |
| [33] |
Altiok E, Becker M, Hamada S, Reith S, Marx N, Hoffmann R. Optimized guidance of percutaneous edge-to edge repair of the mitral valve using real-time 3-D transesophageal echocardiography. Clin Res Cardiol 2011; 100:675-681.
doi: 10.1007/s00392-011-0296-1 pmid: 21369924 |
| [34] |
Melillo F, Fisicaro A, Stella S, Ancona F, Capogrosso C, Ingallina G, et al. Systematic fluoroscopic-echocardiographic fusion imaging protocol for transcatheter edge-to-edge mitral valve repair intraprocedural monitoring. J Am Soc Echocardiogr 2021; 34:604-613.
doi: 10.1016/j.echo.2021.01.010 |
| [35] | Sündermann SH, Biaggi P, Grünenfelder J, Gessat M, Felix C, Bettex D, et al. Safety and feasibility of novel technology fusing echocardiography and fluoroscopy images during MitraClip interventions. EuroIntervention 2014;9:1210-1216. |
| [36] | Navarese EP, Zhang Z, Kubica J, Andreotti F, Farinaccio A, Bartorelli AL, et al. Development and validation of a practical model to identify patients at risk of bleeding after TAVR. JJACC Cardiovasc Interv 2021; 14:1196-1206. |
| [37] |
Jia Y, Luosang G, Li Y, Wang J, Li P, Xiong T, et al. Deep learning in prediction of late major bleeding after transcatheter aortic valve replacement. Clin Epidemiol 2022; 14:9-20.
doi: 10.2147/CLEP.S333147 pmid: 35046728 |
| [38] |
Kwak S, Everett RJ, Treibel TA, Yang S, Hwang D, Ko T, et al. Markers of myocardial damage predict mortality in patients with aortic stenosis. J Am Coll Cardiol 2021; 78:545-558.
doi: 10.1016/j.jacc.2021.05.047 pmid: 34353531 |
| [39] |
Zweck E, Spieker M, Horn P, Iliadis C, Metze C, Kavsur R, et al. Machine learning identifies clinical parameters to predict mortality in patients udergoing transcatheter mitral valve repair. JACC Cardiovasc Interv 2021; 14:2027-2036.
doi: 10.1016/j.jcin.2021.06.039 |
| [40] |
Modine T, Perrin N, Ben Ali W. Trust in machine learning models for mortality prediction following mitral TEER: Are we ready yet? JACC Cardiovasc Interv 2021; 14:2037-2038.
doi: 10.1016/j.jcin.2021.08.002 |
| [41] |
Hernandez-Suarez DF, Kim Y, Villablanca P, Gupta T, Wiley J, Nieves-Rodriguez BG, et al. Machine learning prediction models for in-hospital mortality after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019; 12:1328-1338.
doi: 10.1016/j.jcin.2019.06.013 |
| [42] |
Tse G, Zhou J, Lee S, Liu Y, Leung KSK, Lai RWC, et al. Multi-parametric system for risk stratification in mitral regurgitation: A multi-task Gaussian prediction approach. Eur J Clin Invest 2020; 50:e13321.
doi: 10.1111/eci.v50.11 |
| [43] |
Engelhardt S, Sauerzapf S, Brčić A, Karck M, Wolf I, De Simone R. Replicated mitral valve models from real patients offer training opportunities for minimally invasive mitral valve repair. Interact Cardiovasc Thorac Surg 2019; 29:43-50.
doi: 10.1093/icvts/ivz008 |
| [44] |
Liu J, Al'Aref SJ, Singh G, Caprio A, Moghadam AAA, Jang SJ, et al. An augmented reality system for image guidance of transcatheter procedures for structural heart disease. PloS One 2019; 14:e0219174.
doi: 10.1371/journal.pone.0219174 |
| [1] | V. Merin Shobi, MCA, MPhil , ME, F. Ramesh Dhanaseelan, MSc, MTech , PhD. Review on Image Inpainting using Intelligence Mining Techniques [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 366-372. |
| [2] | Lujing Li, MD, Zuofeng Xu, MD. The Value of VTTQ Combined with B-mode US for Distinguishing Benign from Malignant Breast Masses by Comparing with SE: A Clinical Research [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 394-400. |
| [3] | Wenjun Zhang, MD, Mi Zhou, PhD, Qingguo Meng, MD, Lin Zhang, MS, Xin Liu, MS, Paul Liu, PhD, Dong Liu, PhD. Rapid Screening of Carotid Plaque in Cloud Handheld Ultrasound System Based on 5G and AI Technology [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(2): 152-157. |
| [4] | Jiaojiao Ma, MD, Xinying Jia, MD, Guanghan Li, MD, Dandan Guo, MD, Xuehua Xi, MD, Tongtong Zhou, MD, Ji-Bin Liu, MD, Bo Zhang, MD. Development of 5G-based Remote Ultrasound Education: Current Status and Future Trends [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(2): 197-203. |
| [5] | Won-Chul Bang, PhD, Vice President, Yeong Kyeong Seong, PhD, Jinyong Lee. The Impact of Deep Learning on Ultrasound in Diagnosis and Therapy: Enhancing Clinical Decision Support, Workflow Efficiency, Quantification, Image Registration, and Real-time Assistance [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(2): 204-216. |
| [6] | Mengna He, MD, PHD, Lu Zhu, MD, Tianan Jiang, MD, PHD. Findings of Fat Containing Hepatocellular Carcinoma on Contrast-enhanced Ultrasound with Sonazoid: A Case Report [J]. Advanced Ultrasound in Diagnosis and Therapy, 2021, 5(2): 98-101. |
| [7] | Ningning Niu, MD, Ying Tang, MD, Jingwen Zhao, MD. Real-Time Tissue Elastography: A Noninvasive Technique to Evaluate Liver Damage after Brain Death in Animal Mode [J]. Advanced Ultrasound in Diagnosis and Therapy, 2020, 4(4): 322-328. |
| [8] | Sriharsha Gummadi, MD, Nirmal Patel, Haresh Naringrekar, MD, Laurence Needleman, MD, Andrej Lyshchik, MD PhD, Patrick O’Kane, MD, Jesse Civan, MD, John R Eisenbrey, PhD. Automated Machine Learning in the Sonographic Diagnosis of Non-alcoholic Fatty Liver Disease [J]. Advanced Ultrasound in Diagnosis and Therapy, 2020, 4(3): 176-182. |
| [9] | Lanting Jia, MD, Jiaqi Zhao, MD, Qi Xu, PhD, Qian Pan, MD, Jianquan Zhang, MD. Quantitative Analysis of Textural Features Extracted from Sonograms of Biceps under Different Physiological States [J]. Advanced Ultrasound in Diagnosis and Therapy, 2020, 4(3): 183-188. |
| [10] | Shuo Wang, BS, Ji-Bin Liu, MD, Ziyin Zhu, MD, John Eisenbrey, PhD. Artificial Intelligence in Ultrasound Imaging: Current Research and Applications [J]. Advanced Ultrasound in Diagnosis and Therapy, 2019, 3(3): 53-61. |
| [11] | Ying Wang, MD, Luying Gao, MD, Yuxin Jiang, MD, Hui Pan, MD, Jun Zhao, MA, Xin Zhou, MA, Qiong Wu, MM, Ruyu Liu, MM, Bo Zhang, MD. Image Features-based Learning Effectively Improves Inter-Observer Agreement for Beginners in Evaluating Thyroid Nodule with Ultrasound [J]. Advanced Ultrasound in Diagnosis and Therapy, 2019, 3(1): 1-5. |
| [12] | Wenlong Zeng, Christopher J Krueger, Zhifei Dai, PhD. Ultrasonic Thermal Strain Imaging for Noninvasive Temperature Estimation in Tissue [J]. Advanced Ultrasound in Diagnosis and Therapy, 2018, 2(2): 71-81. |
| [13] | Jinlian Ma, PhD, Dexing Kong, PhD. Deep Learning Models for Segmentation of Lesion Based on Ultrasound Images [J]. Advanced Ultrasound in Diagnosis and Therapy, 2018, 2(2): 82-93. |
| [14] | Jinghua Li, MD, Yuanyuan An, MD, Lina Zhang, MD, Yinghua Xuan, MD, Qingqing Wu, MD. Assessment of the Cervix Using Strain Elastography in Pregnant Women with Spontaneous Preterm Birth [J]. Advanced Ultrasound in Diagnosis and Therapy, 2018, 2(2): 106-112. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Share: WeChat
Copyright ©2018 Advanced Ultrasound in Diagnosis and Therapy
|
 Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
