Advanced Ultrasound in Diagnosis and Therapy ›› 2025, Vol. 9 ›› Issue (3): 229-244.doi: 10.26599/AUDT.2025.240052
• Review Article • Previous Articles Next Articles
Zhang Ronga,b, Xie Litinga,c, Jin Qijinga, Zhang Chengyuea, Guo Tenga, Zhao Qiyua,*( ), Jiang Tian’ana,c,*(
), Jiang Tian’ana,c,*( )
)
Received:2024-09-27
Revised:2024-12-14
Accepted:2025-03-19
Online:2025-09-30
Published:2025-10-13
Contact:
Department of Ultrasound Medicine, First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou, Zhejiang 310003, P.R. China. e-mail: zhaoqiyu2000@zju.edu.cn(QY Zhao); tiananjiang@zju.edu.cn (Tian’an Jiang),
Zhang Rong, Xie Liting, Jin Qijing, Zhang Chengyue, Guo Teng, Zhao Qiyu, Jiang Tian’an. Research Progress on the Application of Irreversible Electroporation Ablation in Cancers. Advanced Ultrasound in Diagnosis and Therapy, 2025, 9(3): 229-244.
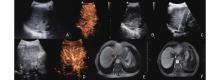
Figure 1
A 66-year-old male patient with a history of “liver cancer and hepatitis B cirrhosis” underwent liver transplantation in 2022. One year later, the liver cancer recurred, despite undergoing TACE, radiotherapy, and targeted therapy, the lesion remained active, leading to IRE treatment. (A) The US lesion prior to surgery was approximately 31×30 mm, located at the second porta hepatis; (B) The lesion showed rapid enhancement at the 20th second during preoperative contrast-enhanced ultrasound (CEUS); (C) Two ablation needles were used during the lesion ablation procedure; (D) Immediate CEUS after ablation showed no significant enhancement of the lesion. MRI imaging during the venous phase showed an abnormal signal focus in segment S7 with unclear boundaries; (E) Preoperative; (F) One month post-surgery."
Table 1
Irreversible electroporation (IRE) clinical data for liver cancer"
| Author (year, reference No) | Patients | Lesions | Mean cancer size (mm) | Median follow-up (months) | Results | Treatment-related complication |
| Thomson et al. (2011, 46) | n = 25 | n = 75 | 25 | 3 | Complete remission rate (CR):colorectal liver metastases (CLM) (RE-CIST) was 50%, hepatocellular carcinoma (HCC) was 83.3%no significant cancer response in lesions ≥ 50 mm | Cardiac rhythm disturbance in 2 patients, pneumothorax (1 patient), brachial plexus neuropraxia related to positioning (2 patients). |
| Philips et al. (2013, 47) | n = 60 | n = 66 | 38 | 18 | A total of 12 patients were felt to have been incompletely ablated. 35 (31%) patients had recurrence. 2 peri-operative deaths. | Among the high grade adverse events (AEs) were 3 cases of deep vein thrombosis (DVT)/pulmonary embolism (PE), 1 of bile leak and 2 of biliary strictures, 2 of bleeding requiring transfusions and 1portal vein thrombus. The attributable high-grade complication rate was 4%. |
| Hosein et al. (2014. 59) | n = 29 | n = 58 | 27 | - | The 2-year progression-free survival (PFS) rate was 18%, the 2-year overall survival (OS) rate was 62%. | Arrhythmias (n = 1) and post-procedure pain (n = 1). |
| Niessen et al. (2016, 48) | n = 34 | n = 65 | 24 | 13.9 | The local recurrence-free survival (LRFS) at 3, 6, and 12 months were 87.4%, 79.8%, and 74.8%. | The overall complication rate was 27.5%, 6 cases being major complications, 8 cases being minor complications. |
| Bhutiani et al. (2016. 51) | n = 30 | n = 30 | 30 | 6 | The success rate for liver cancer reached 97%. | Major and minor complication rates were 27% for patients undergoing IRE ablation. |
| Distelmaier et al. (2017,49) | n = 29 | n = 43 | - | 24 | 40 of the 43 (93%) target cancers were detected achieved complete ablation, only two cases of recurrence within the ablation area. | The blood vessels in the surgical area were well preserved and the blood supply was normal in 29 patients, 5 cases had mild to moderate cholestasis. |
| Frühling et al. (2017. 58) | n = 30 | n = 38 | 24 | 22.3 | Ablation success was 78.9% at 3 months,65.8% at 6 months. | 6 patients (20%) have a minor complication, 1 patient (3.3%) suffered from a major complication (bile duct dilatation and stricture of the portal vein and bile duct). |
| Niessen et al. (2017. 57) | n = 71 | n = 103 | 19 | 35.7 | The median OS was 26.3 months; patients with a cancer diameter >30 mm or more than 2 lesions died significantly earlier than patients with smaller or fewer cancers. | 5 major complications (4 liver abscess, 1 myocardial infarction) and 7 minor complications (2 pneumothorax, 2 cardiac arrhythmia, 3 hematoma). |
| Alnaggar et al. (2018. 53) | nA (IRE) = 20 nB (IRE-NK) = 20 | - | - | 7.6 | The median OS in the group A and group B was 8.9 and 10.1 months. | No severe complications (such as ruptured or hepatic failure, myoglobinuria, or acute renal failure) were reported post-IRE. |
| Yang et al. (2019. 54) | nA (IRE) = 22 nB (IRE-NK) = 18 | - | nA:47.3 nB:48.1 | 14 | PFS: IRE was 10.6 months IRE-NK was 15.1 months. OS: IRE was 17.9 months IRE-NK was 23.2 months. | Pain, pleural effusion, ascites, fatigue, and fever. There was no difference among the two groups. |
| Cornelis et al. (2020. 56) | n = 25 | n = 25 | 21 | 25 | Cancer size (> 20 mm), probe spacing (> 20 mm) and the presence of Myocardial Infarction (MI) were significant predictors of time to local cancer progression (LTP). | No major complications. |
| Meijerink et al. (2021. 55) | n = 51 | n = 76 | 22 | 23.9, 9.5, 6.3 | 1-year LTP-free survival of 68%. Local control following repeat procedures was 74% of participants (37 of 50). Median OS from first IRE was 2.7 years. | Overall complication rate, 40%, 1 participant (2%) had an infected biloma after IRE, died fewer than 90 days after the procedure (grade 5 adverse event). |
| Frühling et al. (2023.50) | n = 149 | n = 206 | - | 58 | Median Survival Time (MST): HCC was 27.0 months, colorectal cancer liver metastasis (CRCLM) was 35.0 months LTP: HCC was 21.0 months, CRCLM was 6.0 months. | During the 30-day postoperative follow-up, 26 (17.4%) patients experienced complications. |
| Hassany et al. (2024.52) | n = 10 | n = 10 | 26.9 | 24 | 5 patients developed recurrence away from the primary ablated site and 2 patients remained alive. | Clinical decompensation occurred in 6 (60%) patients in the IRE group. The two most common causes were ascites and portal vein thrombosis |
Table 2
Irreversible electroporation (IRE) clinical data for pancreatic cancer"
| Author (year, reference No) | Patients | Lesions | Mean cancer size (mm) | Median follow-up (months) | Results | Treatment-related complication |
| Martin et al. (2013. 64) | n = 54 | n = 54 | 32 | 10 | Local progression-free survival (PFS) was 14 months, distant PFS was 15 months, overall survival (OS) was 20 months | No major complications. |
| Yan et al. (2016. 62) | n = 25 | n = 25 | 42 | 3 | The overall rate of stable disease was 28%, 36% achieved partial response, and lower serum CA19-9 levels were recorded in all patients at discharge. | 4 intraoperative procedure-related complications. 3 Grade A pancreatic fistulas, 3 delayed gastric emptying, 1 acute pancreatitis, 1 upper gastrointestinal hemorrhage, and 1 portal vein thrombosis |
| Scheffer et al. (2017. 63) | n = 25 | n = 25 | 40 | 12 | Median event-free survival (EFS) was 8 months; The median OS was 11 months from IRE. | 12 minor complications (grade I or II) ,11 major complications (9 grade III, 2 grade IV) in 10 patients. |
| Narayanan et al. (2017. 65) | n = 50 | n = 50 | 32 | - | The median OS was 14.2 months after IRE. cancers ≤ 30 mm (n = 24) with > 30 mm (n = 26): 16.2 vs 9.9 months | 7 abdominal pain,1 pancreatitis, 1 sepsis, 1 gastric leak |
| Lin et al. (2017. 67) | nA (IRE) = 30 nB (IRE-NK) = 37 | n = 67 | - | 7.9 | The median PFS and OS of the IRE-NK group was higher than the IRE group. The median PFS/OS of multiple IRE-NK group was higher than single IRE-NK group (9.9 vs 8.2 months)/ (13.7 vs12.1 months). | Cough 12.7%, nausea and emesis 6.8%, pain of puncture point 25.3%, duodenum and gastric retention 5.9%, fatigue 21.5, fever 33.5%, blood pressure intraoperative transient reduction 27.4% and white cell count reduction 22.6% |
| Ruarus et al. (2020. 60) | n = 50 | n = 61 | 40 | - | Local recurrence developed in 23 of the 50 participants after IRE. For locally advanced pancreatic cancer (LAPC) patients median OS was 10 months from IRE. For participants with postresection local recurrence, the median OS was 9 months from IRE. | 14 minor and 21 major complications occurred in 29 of the 50 participants (58%). One of two participants who died may have been linked to IRE. |
| Li et al. (2020. 61) | n = 64 | n = 64 | - | 29.3 | The median OS and PFS of the entire cohort was 24.6 months and 12.0 months. | There were 7, 4, and 2 cases of pancreatic fistula, incision infection, and upper gastrointestinal bleeding. |
| Lin et al. (2020. 68) | nA (IRE+γδ T-cell) = 30 nB (IRE) =32 | - | nA:40 nB:39 | 12 | OS: group A vs group B:14.5 months vs. 11 months Median PFS: group A vs group B:11 months vs. 8.5 months. | 14 major and 25 minor AEs happened in 31 of the 62 patients (50%), including 16 participants in group A and 15 in group B. |
| O'Neill et al. (2020. 69) | n = 10 | n = 10 | - | 12 | Mean time to progression was 6.3 months, median overall survival of 18.0 months. | Seven patients developed grade 3/4 treatment-related adverse events. |
| Pan et al. (2020. 70) | nA (IRE) = 46 nB (IRE-NK) = 46 | n = 92 | nA:41 nB:44 | 6-29 | Disease-free survival (DFS) and OS between the IRE and IRE-NK group (6.1 ± 3.9 months vs. 7.2 ± 4.3 months, and 11.8 ± 4.6 months vs. 12.4 ± 5.2 months) | No major complications. |
| Ma et al. (2023.71) | nA (IRE) = 25 nB (IRE-NK) = 78 | n = 103 | nA:41 nB:38 | 18.2 | Group A (IRE in combination with chemotherapy and programmed death protein-1/Programmed Death-Ligand 1 (PD-1/PD-L1) blockade) compared to group B (IRE in combination with chemotherapy): median OS: 23.6 vs 19.4 months, median PFS: 18.2 vs 14.7 months. | Immune-related AEs in group A were pruritus (24%), hypothyroidism (16%), increased bilirubin (16%), ALT increase (16%). The major (grade 3 - 4) IRE-related AEs were cardiac arrhythmias (48%), hypertension (48%), pancreatitis (28%), hemorrhage (16%). |
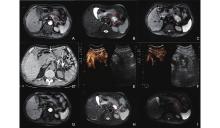
Figure 2
A 74-year-old male patient diagnosed with locally advanced pancreatic cancer (LAPC) presented with involvement of the body and tail of the pancreas, accompanied by gastric wall invasion, persisting for over five months. The patient exhibited advanced pancreatic cancer with distant metastasis, resulting in the loss of surgical intervention opportunities and failure of the initial chemotherapy regimen. Interventional sonographers and radiologists conducted ultrasound-guided, CT-assisted percutaneous irreversible electroporation (IRE) ablation for the cancers located in the pancreatic body and tail, administered under general anesthesia. A contrast-enhanced MRI of the pancreas was performed prior to the IRE procedure (images A, B, C). (A) T1-weighted image in the arterial phase shows a long T1 signal nodule (red circle) in the body and tail of the pancreas; (B) T2-weighted image shows that the lesion is a long T2 signal nodule (red circle); (C) DWI shows increased signal in the lesion (red circle). Intraoperative CT monitoring of needle placement combined with contrast-enhanced ultrasound to determine ablation range (images D, E, F). (D) The second and third IRE ablation needles are located on both sides below the lesion; (E) Preoperative contrast-enhanced ultrasound shows that the lesions in the pancreatic body and tail show lack of blood supply; (F) Immediate postoperative contrast-enhanced ultrasound shows that the pancreatic body A large non-enhancement area in the tail covers the target cancer, indicating complete ablation. Contrast-enhanced MRI of the pancreas 1 month after IRE (images G, H, I). (G) T1-weighted image in the arterial phase shows a long T1 signal nodule in the body and tail of the pancreas (red circle); (H) T2-weighted image shows that the lesion is a long T2 signal nodule (red circle); (I) DWI shows no abnormality in the signal of the lesion (red circle)."
Table 3
Irreversible electroporation (IRE) clinical data for prostate cancer and kidney cancer"
| Author (year, reference No) | Patients | Lesions | Mean cancer size | Median follow-up | Results | Treatment-related complication |
| Prostate cancers | ||||||
| Onik et al. (2010. 72) | n = 16 | n = 16 | - | 3 weeks | All patients were continent immediately, 15 patients had no evidence for cancer in the ablation area. 1 patient has microfocus of Gleason 6 lesion outside ablation area. | No major complications. |
| Fenner et al. (2014. 77) | n = 34 | n = 34 | - | 6 months | 19 patients retain erectile function. The patients who can control urination independently before retain this ability after surgery. | 2 cases of urinary retention, 6 cases of hematuria, 5 cases of dysuria, and 5 cases of urinary tract infection. |
| Ting et al. (2016. 73) | n = 25 | n = 25 | - | 8 months | No suspicious findings on mp-MRI (n = 24) or biopsy (n = 21) in all patients. 4 significant finding on biopsy adjacent to the treatment zone, and 1 in outfield. | 1 Clavien Grade 3 complication and five Grade 1 complications. Functional follow-up confirmed no significant change in sexual or bowel function. |
| Dong et al. (2018. 76) | n = 50 | n = 50 | - | 6 months | The ablation margin is obvious, the prostate capsule and urethra were intact. | No major complications. |
| Wang et al. (2022. 74) | n = 109 | n = 109 | 38.1 mL | 6 months | Median (IQR) international prostate symptom score was 4.5 (2.0 - 9.5), and median (IQR) international index of erectile function 5 score was 2.0 (0.5 - 12.5). | The overall complication rate was 37.6%, like elevated white blood cell level in urine, followed by epididymiti, prolonged gross hematuria, urinary retention, urinary tract infection and bladder stones. |
| Scheltema et al. (2023. 75) | n = 229 | n = 229 | - | 60 months | Failure-free survival (FFS) rates were 91% at 3 years, 84% at 5 years and 69% at 8 years. Metastasis-free survival was 99.6%, prostate cancer (PC) a-specific and overall survival were 100%. | Short-term urinary continence was preserved (98%, 3 of 144 at baseline, 99%, 1 of 131 at 12 months) and erections sufficient for intercourse decreased by 13% compared to baseline (71% to 58%). |
| Kidney cancers | ||||||
| Buijs et al. (2019. 80) | n = 10 | n = 10 | 23 mm | 6 months | Technical success was achieved in 9 cases. | 1 patient had a grade 3 Clavien-Dindo complication. |
| Wah et al. (2021. 78) | n = 26 | n = 30 | 25 mm | 37 months | 22 renal cell carcinomas (RCCs) were completely IRE ablated. The overall 2- and 3-year cancer-specific (CS), local recurrence-free (LRF) and metastasis-free (MF) survival rates are 89%, 96%, 91% and 87%. | 1 patient had CD-III complication with a proximal ureteric injury.5 patients developed > 25% reduction of estimated glomerular filtration rate (eGFR). No patient need requirement for renal dialysis. |
| Wang et al. (2021.79) | n = 15 | n = 19 | 24 mm | 6 months | The success rate of the procedure was 100%. | 2 patients had transient gross hematuria. Self-limiting perinephric hematomas occurred in 1 patient. |
| [1] | Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membrane Biol 1972; 10: 279-290. |
| [2] | Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim Biophys Acta 1982; 694: 227-277. |
| [3] | Weaver JC. Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem 1993; 51: 426-435. |
| [4] | Freeman SA, Wang MA, Weaver JC. Theory of electroporation of planar bilayer membranes: predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys J 1994; 67: 42-56. |
| [5] | Geboers B, Scheffer HJ, Graybill PM, Ruarus AH, Nieuwenhuizen S, Puijk RS, et al. High-voltage electrical pulses in oncology: irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy. Radiology 2020; 295: 254-272. |
| [6] | Novickij V, Malyško V, Želvys A, Balevičiūtė A, Zinkevičienė A, Novickij J, et al. Electrochemotherapy using doxorubicin and nanosecond electric field pulses: a pilot in vivo study. Molecules 2020; 25: 4601. |
| [7] | Qi Y, Sun D, Wang L, Yu J, Liang P. State-of-the-art and development trend of interventional ultrasound in China. Advanced Ultrasound in Diagnosis and Therapy 2023; 7: 313-320. |
| [8] | Batista Napotnik T, Polajžer T, Miklavčič D. Cell death due to electroporation - a review. Bioelectrochemistry 2021; 141: 107871. |
| [9] | Michel O, Pakhomov AG, Casciola M, Saczko J, Kulbacka J, Pakhomova ON. Electropermeabilization does not correlate with plasma membrane lipid oxidation. Bioelectrochemistry 2020; 132: 107433. |
| [10] | Maccarrone M, Bladergroen MR, Rosato N, Finazzi Agrò AF. Role of lipid peroxidation in electroporation-induced cell permeability. Biochem Bioph Res Co 1995; 209: 417-425. |
| [11] | Kotnik T, Miklavcic D. Theoretical evaluation of voltage inducement on internal membranes of biological cells exposed to electric fields. Biophys J 2006; 90: 480-491. |
| [12] | Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane electroporation and electropermeabilization: mechanisms and models. Annu rev biophys 2019; 48: 63-91. |
| [13] | Ringel-Scaia VM, Beitel-White N, Lorenzo MF, Brock RM, Huie KE, Coutermarsh-Ott S, et al. High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity. EBioMedicine 2019; 44: 112-125. |
| [14] | Mi Y, Sun C, Yao C, Xiong L, Wang S, Luo X, et al. [Effect of steep pulsed electric fields on the immune response of tumor-bearing Wistar mice]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2007; 24: 253-256. |
| [15] | Mi Y, Sun C, Yao C, Xiong L, Wang S, Li C, et al. Lethal effects of Steep Pulsed Electric Field (SPEF) to Target Lymphatic Capillaries in VX2Implanted Breast Cancer of Rabbits. Conf Proc IEEE Eng Med Biol Soc 2005; 2005: 4904-4907. |
| [16] | Bulvik BE, Rozenblum N, Gourevich S, Ahmed M, Andriyanov AV, Galun E, et al. , Irreversible electroporation versus radiofrequency ablation: a comparison of local and systemic effects in a small-animal model. Radiology 2016; 280: 413-424. |
| [17] | Imran KM, Nagai-Singer MA, Brock RM, Alinezhadbalalami N, Davalos RV, Allen IC. Exploration of novel pathways underlying irreversible electroporation induced anti-tumor immunity in pancreatic cancer. Front Oncol 2022; 12: 853779. |
| [18] | Zhang N, Li Z, Han X, Zhu Z, Li Z, Zhao Y, et al. Irreversible electroporation: an emerging immunomodulatory therapy on solid tumors. Front Immunol 2021; 12: 811726. |
| [19] | Yao C, Lv Y, Dong S, Zhao Y, Liu H. Irreversible electroporation ablation area enhanced by synergistic high- and low-voltage pulses. PLoS One 2017; 12: e0173181. |
| [20] | Weaver JC, Smith KC, Esser AT, Son RS, Gowrishankar TR. A brief overview of electroporation pulse strength-duration space: a region where additional intracellular effects are expected. Bioelectrochemistry 2012; 87: 236-243. |
| [21] | Jiang C, Qin Z, Bischof J. Membrane-targeting approaches for enhanced cancer cell destruction with irreversible electroporation. Ann Biomed Eng 2014; 42: 193-204. |
| [22] | Li C, Yao C, Sun C, Guo F, Zhou W, Xiong Z. Dependence on electric field intensities of cell biological effects induced by microsecond pulsed electric fields. IEEE Trans Dielectr Electr Insul 2011; 18: 2083-2088. |
| [23] | Sano MB, Neal RE 2nd, Garcia PA, Gerber D, Robertson J, Davalos RV. Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed Eng Online 2010; 9: 83. |
| [24] | Mi Y, Xu J, Liu Q, Wu X, Zhang Q, Tang J. Single-cell electroporation with high-frequency nanosecond pulse bursts: simulation considering the irreversible electroporation effect and experimental validation. Bioelectrochemistry 2021; 140: 107822. |
| [25] | Peng W, Cao Y, Zhang Y, Zhong A, Zhang C, Wei Z, et al. Optimal irreversible electroporation combined with nano-enabled immunomodulatory to boost systemic antitumor immunity. Adv Healthc Mater 2024; 13: e2302549. |
| [26] | Sano MB, Fesmire CC, DeWitt MR, Xing L. Burst and continuous high frequency irreversible electroporation protocols evaluated in a 3D tumor model. Phys Med Biol 2018; 63: 135022. |
| [27] | Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005; 33: 223-231. |
| [28] | Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, et al. Tumor ablation with irreversible electroporation. PLoS One 2007; 2: e1135. |
| [29] | Guo Y, Zhang Y, Klein R, Nijm GM, Sahakian AV, Omary RA, et al. Irreversible electroporation therapy in the liver: longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer Res 2010; 70: 1555-1563. |
| [30] | Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology 2010; 255: 426-433. |
| [31] | Bower M, Sherwood L, Li Y, Martin R. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol 2011; 104: 22-28. |
| [32] | Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat 2007; 6: 295-300. |
| [33] | Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat 2007; 6: 307-312. |
| [34] | Kim HB, Sung CK, Baik KY, Moon KW, Kim HS, Yi JH, et al. Changes of apoptosis in tumor tissues with time after irreversible electroporation. Biochem Biophys Res Commun 2013; 435: 651-656. |
| [35] | Liu Y, Xiong Z, Zhou W, Hua Y, Li C, Yao C. Percutaneous ultrasound-guided irreversible electroporation: a goat liver study. Oncol Lett 2012; 4: 450-454. |
| [36] | Jeon SM, Davaa E, Jiang Y, Jenjob R, Truong NT, Shin KJ, et al. Assessment of hepatic lesions after non-thermal tumor ablation by irreversible electroporation in a pig model. Technol Cancer Res Treat 2023; 22: 15330338221147122. |
| [37] | Sorokin I, Canvasser N, Johnson B, Lucas E, Cadeddu JA. Irreversible electroporation for renal ablation does not cause significant injury to adjacent ureter or bowel in a porcine model. J Endourol 2021; 35: 873-877. |
| [38] | Olweny EO, Kapur P, Tan YK, Park SK, Adibi M, Cadeddu JA. Irreversible electroporation: evaluation of nonthermal and thermal ablative capabilities in the porcine kidney. Urology 2013; 81: 679-684. |
| [39] | Yan L, Liang B, Feng J, Zhang HY, Chang HS, Liu B, et al. Safety and feasibility of irreversible electroporation for the pancreatic head in a porcine model. World J Gastrointest Oncol 2022; 14: 1499-1509. |
| [40] | Lee EW, Shahrouki P, Peterson S, Tafti BA, Ding PX, Kee ST. Safety of irreversible electroporation ablation of the pancreas. Pancreas 2021; 50: 1281-1286. |
| [41] | Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun 2019; 10: 899. |
| [42] | Blazevski A, Scheltema MJ, Amin A, Thompson JE, Lawrentschuk N, Stricker PD. Irreversible electroporation (IRE): a narrative review of the development of IRE from the laboratory to a prostate cancer treatment. Bju Int 2020; 125: 369-378. |
| [43] | Phillips MA, Narayan R, Padath T, Rubinsky B. Irreversible electroporation on the small intestine. Brit J Cancer 2012; 106: 490-495. |
| [44] | Garcia PA, Rossmeisl JH Jr, Robertson J, Ellis TL, Davalos RV. Pilot study of irreversible electroporation for intracranial surgery. Annu Int Conf IEEE Eng Med Biol Soc 2009; 2009: 6513-6516. |
| [45] | Thomson K. Human experience with irreversible electroporation, in irreversible electroporation. Springer Berlin Heidelberg: Berlin, Heidelberg 2010; 249-254. |
| [46] | Thomson K, Kee ST. Clinical research on irreversible electroporation of the liver, in clinical aspects of electroporation. Springer New York: New York, NY 2011; 237-246. |
| [47] | Philips P, Hays D, Martin RC. Irreversible electroporation ablation (IRE) of unresectable soft tissue tumors: learning curve evaluation in the first 150 patients treated. PLoS One 2013; 8: e76260. |
| [48] | Niessen C, Beyer LP, Pregler B, Dollinger M, Trabold B, Schlitt HJ, et al. Percutaneous ablation of hepatic tumors using irreversible electroporation: a prospective safety and midterm efficacy study in 34 patients. J Vasc Interv Radiol 2016; 27: 480-486. |
| [49] | Distelmaier M, Barabasch A, Heil P, Kraemer NA, Isfort P, Keil S, et al. Midterm safety and efficacy of irreversible electroporation of malignant liver tumors located close to major portal or hepatic veins. Radiology 2017; 285: 1023-1031. |
| [50] | Frühling P, Stillström D, Holmquist F, Nilsson A, Freedman J. Irreversible electroporation of hepatocellular carcinoma and colorectal cancer liver metastases: a nationwide multicenter study with short- and long-term follow-up. Eur J Surg Oncol 2023; 49: 107046. |
| [51] | Bhutiani N, Philips P, Scoggins CR, McMasters KM, Potts MH, Martin RC. Evaluation of tolerability and efficacy of irreversible electroporation (IRE) in treatment of Child-Pugh B (7/8) hepatocellular carcinoma (HCC). Hpb 2016; 18: 593-599. |
| [52] | Hassany M, Mahboub AM, Mostafa W, Debian H, Shousha HI, El-Serafy M. Assessment of efficacy and safety of irreversible electroporation versus TACE for treatment of difficult location hepatocellular carcinoma. Egypt Liver J 2024; 14: 1-11. |
| [53] | Alnaggar M, Lin M, Mesmar A, Liang S, Qaid A, Xu K, et al. Allogenic natural killer cell immunotherapy combined with irreversible electroporation for stage IV hepatocellular carcinoma: survival outcome. Cell Physiol Biochem 2018; 48: 1882-1893. |
| [54] | Yang Y, Qin Z, Du D, Wu Y, Qiu S, Mu F, et al. Safety and short-term efficacy of irreversible electroporation and allogenic natural killer cell immunotherapy combination in the treatment of patients with unresectable primary liver cancer. Cardiovasc Inter Rad 2019; 42: 48-59. |
| [55] | Meijerink M. R., etal. Irreversible Electroporation to Treat Unresectable Colorectal Liver Metastases (COLDFIRE-2): A Phase II, Two-Center, Single-Arm Clinical Trial. Radiology, 2021; 299 2 470-480. |
| [56] | Meijerink MR, Ruarus AH, Vroomen LGPH, Puijk RS, Geboers B, Nieuwenhuizen S, et al. Peri-tumoral metallic implants reduce the efficacy of irreversible electroporation for the ablation of colorectal liver metastases. Cardiovasc Inter Rad 2020; 43: 84-93. |
| [57] | Niessen C, Thumann S, Beyer L, Pregler B, Kramer J, Lang S, et al. Percutaneous irreversible electroporation: long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci Rep 2017; 7: 43687. |
| [58] | Frühling P, Nilsson A, Duraj F, Haglund U, Norén A. Single-center nonrandomized clinical trial to assess the safety and efficacy of irreversible electroporation (IRE) ablation of liver tumors in humans: short to mid-term results. Ejso-eur J Surg Onc 2017; 43: 751-757. |
| [59] | Hosein PJ, Echenique A, Loaiza-Bonilla A, Froud T, Barbery K, Rocha Lima CM, et al. Percutaneous irreversible electroporation for the treatment of colorectal cancer liver metastases with a proposal for a new response evaluation system. J Vasc Interv Radiol 2014; 25: 1233-1239. |
| [60] | Ruarus AH, Vroomen LGPH, Geboers B, van Veldhuisen E, Puijk RS, Nieuwenhuizen S, et al. Percutaneous irreversible electroporation in locally advanced and recurrent pancreatic cancer (PANFIRE-2): a multicenter, prospective, single-arm, phase II study. Radiology 2020; 294: 212-220. |
| [61] | Li SP, He CB, Wang J, Mao YZ, Lao XM, Cui BK, et al. [Combining intraoperative ultrasound-guided irreversible electroporation with chemotherapy for treating locally advanced pancreatic cancer: a clinical report of 64 cases]. Zhonghua Wai Ke Za Zhi 2020; 58: 787-792. |
| [62] | Yan L, Chen YL, Su M, Liu T, Xu K, Liang F, et al. A single-institution experience with open irreversible electroporation for locally advanced pancreatic carcinoma. Chinese Med J-Peking 2016; 129: 2920-2925. |
| [63] | Scheffer HJ, Vroomen LG, de Jong MC, Melenhorst MC, Zonderhuis BM, Daams F, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: results of the phase I/II PANFIRE study. Radiology 2017; 282: 585-597. |
| [64] | Martin RC 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 2013; 20 suppl 3: S443-449. |
| [65] | Narayanan G, Hosein PJ, Beulaygue IC, Froud T, Scheffer HJ, Venkat SR, et al. Percutaneous image-guided irreversible electroporation for the treatment of unresectable, locally advanced pancreatic adenocarcinoma. J Vasc Interv Radiol 2017; 28: 342-348. |
| [66] | Narayanan G, Bilimoria MM, Hosein PJ, Su Z, Mortimer KM, Martin RCG 2nd. Multicenter randomized controlled trial and registry study to assess the safety and efficacy of the NanoKnife® system for the ablation of stage 3 pancreatic adenocarcinoma: overview of study protocols. BMC Cancer 2021; 21: 785. |
| [67] | Lin M, Liang S, Wang X, Liang Y, Zhang M, Chen J, et al. Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (stage III/IV) pancreatic cancer: a promising treatment. J Cancer Res Clin 2017; 143: 2607-2618. |
| [68] | Lin M, Zhang X, Liang S, Luo H, Alnaggar M, Liu A, et al. Irreversible electroporation plus allogenic Vγ9Vδ2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients. Signal Transduct Tar 2020; 5: 215. |
| [69] | O'Neill C, Hayat T, Hamm J, Healey M, Zheng Q, Li Y, et al. A phase 1b trial of concurrent immunotherapy and irreversible electroporation in the treatment of locally advanced pancreatic adenocarcinoma. Surgery 2020; 168: 610-616. |
| [70] | Pan Q, Hu C, Fan Y, Wang Y, Li R, Hu X. Efficacy of irreversible electroporation ablation combined with natural killer cells in treating locally advanced pancreatic cancer. J Buon 2020; 25: 1643-1649. |
| [71] | Ma Y, Xing Y, Li H, Yuan T, Liang B, Li R, et al. Irreversible electroporation combined with chemotherapy and PD-1/PD-L1 blockade enhanced antitumor immunity for locally advanced pancreatic cancer. Front Immunol 2023; 14: 1193040. |
| [72] | Onik G, Rubinsky B. Irreversible electroporation: first patient experience focal therapy of prostate cancer, in irreversible electroporation. Springer Berlin Heidelberg: Berlin, Heidelberg 2010; 235-247. |
| [73] | Ting F, Tran M, Böhm M, Siriwardana A, Van Leeuwen PJ, Haynes AM, et al. Focal irreversible electroporation for prostate cancer: functional outcomes and short-term oncological control. Prostate Cancer Prostatic Dis 2016; 19: 46-52. |
| [74] | Wang H, Xue W, Yan W, Yin L, Dong B, He B, et al. Extended focal ablation of localized prostate cancer with high-frequency irreversible electroporation: a nonrandomized controlled trial. JAMA Surg 2022; 157: 693-700. |
| [75] | Scheltema MJ, Geboers B, Blazevski A, Doan P, Katelaris A, Agrawal S, et al. Median 5-year outcomes of primary focal irreversible electroporation for localised prostate cancer. Bju Int 2023; 131 suppl 4: 6-13. |
| [76] | Dong S, Wang H, Zhao Y, Sun Y, Yao C. First human trial of high-frequency irreversible electroporation therapy for prostate cancer. Technol Cancer Res Treat 2018; 17: 1533033818789692. |
| [77] | Fenner A. Prostate cancer: irreversible electroporation is a safe and feasible option for focal therapy. Nat Rev Urol 2014; 11: 600. |
| [78] | Wah TM, Lenton J, Smith J, Bassett P, Jagdev S, Ralph C, et al. Irreversible electroporation (IRE) in renal cell carcinoma (RCC): a mid-term clinical experience. Eur Radiol 2021; 31: 7491-7499. |
| [79] | Wang Z, Lu J, Huang W, Wu Z, Gong J, Wang Q, et al. A retrospective study of CT-guided percutaneous irreversible electroporation (IRE) ablation: clinical efficacy and safety. BMC Cancer 2021; 21: 124. |
| [80] | Buijs M, Zondervan PJ, de Bruin DM, van Lienden KP, Bex A, van Delden OM. Feasibility and safety of irreversible electroporation (IRE) in patients with small renal masses: results of a prospective study. Urol Oncol 2019; 37: 183.e1-183.e8. |
| [81] | Ben-David E, Ahmed M, Faroja M, Moussa M, Wandel A, Sosna J, et al. Irreversible electroporation: treatment effect is susceptible to local environment and tissue properties. Radiology 2013; 269: 738-747. |
| [82] | Rai ZL, Feakins R, Pallett LJ, Manas D, Davidson BR. Irreversible electroporation (IRE) in locally advanced pancreatic cancer: a review of current clinical outcomes, mechanism of action and opportunities for synergistic therapy. J Clin Med 2021; 10: 1609. |
| [83] | Srimathveeravalli G, Wimmer T, Silk M, Sofocleous CT, Erinjeri JP, Solomon SB. Treatment planning considerations for IRE in the lung: placement of needle electrodes is critical. J Vasc Interv Radiol 2013; 24: S22. |
| [84] | Fuhrmann I, Probst U, Wiggermann P, Beyer L. Navigation systems for treatment planning and execution of percutaneous irreversible electroporation. Technol Cancer Res Treat 2018; 17: 1533033818791792. |
| [85] | Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K, et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Asses 2013; 17: 1-243. |
| [86] | Appelbaum L, Ben-David E, Sosna J, Nissenbaum Y, Goldberg SN. US findings after irreversible electroporation ablation: radiologic-pathologic correlation. Radiology 2012; 262: 117-125. |
| [87] | Schmidt CR, Shires P, Mootoo M. Real-time ultrasound imaging of irreversible electroporation in a porcine liver model adequately characterizes the zone of cellular necrosis. Hpb 2012; 14: 98-102. |
| [88] | Lu MD, Yu XL, Li AH, Jiang TA, Chen MH, Zhao BZ, et al. Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 2007; 33: 1736-1749. |
| [89] | Zhang GY, Tang Y, Wang MY, Kong WN. Diagnostic utility of superb microvascular imaging of ultrasound examinations to evaluate hepatic ischemia-reperfusion injury. Advanced Ultrasound in Diagnosis and Therapy 2024; 8: 15-21. |
| [90] | Mahmoud O, Makkena A, Wessner CE, Liu JB, Eisenbrey JR, Lyshchik A. Contrast-Enhanced Ultrasound LI-RADS: a pictorial review. Advanced Ultrasound in Diagnosis and Therapy 2023; 7: 321-332. |
| [91] | Ball C, Thomson KR, Kavnoudias H. Irreversible electroporation: a new challenge in "out of operating theater" anesthesia. Anesth Analg 2010; 110: 1305-1309. |
| [92] | Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, et al. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat 2013; 12: 233-241. |
| [93] | Guo Y, Zhang Y, Nijm GM, Sahakian AV, Yang GY, Omary RA, et al. Irreversible electroporation in the liver: contrast-enhanced inversion-recovery MR imaging approaches to differentiate reversibly electroporated penumbra from irreversibly electroporated ablation zones. Radiology 2011; 258: 461-468. |
| [94] | Wendler JJ, Pech M, Porsch M, Janitzky A, Fischbach F, Buhtz P, et al. Urinary tract effects after multifocal nonthermal irreversible electroporation of the kidney: acute and chronic monitoring by magnetic resonance imaging, intravenous urography and urinary cytology. Cardiovasc Inter Rad 2012; 35: 921-926. |
| [95] | Wagstaff PG, Buijs M, van den Bos W, de Bruin DM, Zondervan PJ, de la Rosette JJ, et al. Irreversible electroporation: state of the art. Onco Targets Ther 2016; 9: 2437-2446. |
| [96] | Al-Sakere B, Bernat C, Andre F, Connault E, Opolon P, Davalos RV, et al. A study of the immunological response to tumor ablation with irreversible electroporation. Technol Cancer Res Treat 2007; 6: 301-306. |
| [97] | Narayanan JSS, Ray P, Hayashi T, Whisenant TC, Vicente D, Carson DA, et al. Irreversible electroporation combined with checkpoint blockade and TLR7 stimulation induces antitumor immunity in a murine pancreatic cancer model. Cancer Immunol Res 2019; 7: 1714-1726. |
| [98] | Burbach BJ, O'Flanagan SD, Shao Q, Young KM, Slaughter JR, Rollins MR, et al. Irreversible electroporation augments checkpoint immunotherapy in prostate cancer and promotes tumor antigen-specific tissue-resident memory CD8+ T cells. Nat Commun 2021; 12: 3862. |
| [99] | Neal RE, Davalos RV. The feasibility of irreversible electroporation for the treatment of breast cancer and other heterogeneous systems. Ann Biomed Eng 2009; 37: 2615-2625. |
| [100] | Niessen C, Igl J, Pregler B, Beyer L, Noeva E, Dollinger M, et al. , Factors associated with short-term local recurrence of liver cancer after percutaneous ablation using irreversible electroporation: a prospective single-center study. J Vasc Interv Radiol 2015; 26: 694-702. |
| [101] | Spiliopoulos S, Reppas L, Filippiadis D, Delvecchio A, Conticchio M, Memeo R, et al. Irreversible electroporation for the management of pancreatic cancer: Current data and future directions. World J Gastroentero 2023; 29: 223-231. |
| [102] | Faroja M, Ahmed M, Appelbaum L, Ben-David E, Moussa M, Sosna J, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology 2013; 266: 462-470. |
| [103] | Timmer FEF, Geboers B, Ruarus AH, Schouten EAC, Nieuwenhuizen S, Puijk RS, et al. Irreversible electroporation for locally advanced pancreatic cancer. Tech Vasc Interv Rad 2020; 23: 100675. |
| [104] | Tasu JP, Tougeron D, Rols MP. Irreversible electroporation and electrochemotherapy in oncology: state of the art. Diagn Interv Imaging 2022; 103: 499-509. |
| [105] | Martin RC 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg 2015; 262: 486-494. |
| [106] | Martin RC 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, et al. Early nontumorous CT findings after irreversible electroporation of locally advanced pancreatic cancer. Abdom Radiol 2016; 41: 2142-2149. |
| [107] | Tian G, Liu X, Zhao Q, Xu D, Jiang T. Irreversible electroporation in patients with pancreatic cancer: how important is the new weapon? Biomed Res Int 2018; 2018: 5193067. |
| [108] | Forde PF, Sadadcharam M, Bourke MG, Conway TA, Guerin SR, de Kruijf M, et al. Preclinical evaluation of an endoscopic electroporation system. Endoscopy 2016; 48: 477-483. |
| [109] | Ueshima E, Schattner M, Mendelsohn R, Gerdes H, Monette S, Takaki H, et al. Transmural ablation of the normal porcine common bile duct with catheter-directed irreversible electroporation is feasible and does not affect duct patency. Gastrointest Endosc 2018; 87: 300.e1-300.e6. |
| [110] | Kodama H, Vroomen LG, Ueshima E, Reilly J, Brandt W, Paluch LR, et al. Catheter-based endobronchial electroporation is feasible for the focal treatment of peribronchial tumors. J Thorac Cardiov Sur 2018; 155: 2150-2159. |
| [111] | Ren F, Li Q, Hu L, Yan X, Gao Z, Zhang J, et al. Safety and efficacy of magnetic anchoring electrode-assisted irreversible electroporation for gastric tissue ablation. Surg Endosc 2020; 34: 580-589. |
| [112] | Arena CB, Szot CS, Garcia PA, Rylander MN, Davalos RV. A three-dimensional in vitro tumor platform for modeling therapeutic irreversible electroporation. Biophys J 2012; 103: 2033-2042. |
| [113] | Edd JF, Davalos RV. Mathematical modeling of irreversible electroporation for treatment planning. Technol Cancer Res Treat 2007; 6: 275-286. |
| [114] | Pavliha D, Kos B, Marčan M, Zupanič A, Serša G, Miklavčič D. Planning of electroporation-based treatments using web-based treatment-planning software. J Membrane Biol 2013; 246: 833-842. |
| [115] | Kos B, Voigt P, Miklavcic D, Moche M. Careful treatment planning enables safe ablation of liver tumors adjacent to major blood vessels by percutaneous irreversible electroporation (IRE). Radiol Oncol 2015; 49: 234-241. |
| [1] | Zhang Minyu, Jia Baocheng, Huang Liuming. Imaging Diagnosis of Neonatal Umbilical Arteriovenous Malformation Complicated with Portosystemic Shunt [J]. Advanced Ultrasound in Diagnosis and Therapy, 2025, 9(2): 224-227. |
| [2] | An Zichen, Li Fan. Advancements in the Application of Convolutional Neural Networks in Ultrasound Imaging for Breast Cancer Diagnosis and Treatment [J]. Advanced Ultrasound in Diagnosis and Therapy, 2025, 9(1): 21-31. |
| [3] | Li Tiantian, Zhu Miao, Shen Dejuan, Qian Xiaoqin. The Diagnostic Pitfall of Lymphadenopathy: Ultrasonic Imaging Findings in a Case of Cat Scratch Lymphadenitis [J]. Advanced Ultrasound in Diagnosis and Therapy, 2025, 9(1): 92-95. |
| [4] | Xu Jiale, Xia Shujun, Hua Qing, Mei Zihan, Hou Yiqing, Wei Minyan, Lai Limei, Yang Yixuan, Zhou Jianqiao. Performance of ChatGPT and Radiology Residents on Ultrasonography Board-Style Questions [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(4): 250-254. |
| [5] | Ge Xifeng, Liu Wenzheng, Chen Wen, Mei Fang, Cui Ligang. Gastroduodenal Intussusception Due to a Gastric Gastrointestinal Stromal Tumor in Adult from Sonographer's Perspective [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(4): 255-258. |
| [6] | Shanqing Li, MM, Rong Hu, MM, Xijing Liu, MD, Fan Yang, MD. Update on the Genetics and Prenatal Ultrasound Features of Williams-Beuren Syndrome [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(3): 79-85. |
| [7] | Guiwu Chen, MS, Zhizhong He, BS, Wenqin Liu, MS, Junjun Chen, BS, Xiaomin Liao, MS, Yuhuan Xie, BS. Multimodal Sonographic Findings of Embryonal Carcinoma in the Testis: A Case Report and Literature Review [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(2): 74-77. |
| [8] | Siqi Zheng, MM, Min Bai, MM. Application Progress of Ultrasound Elastography in the Evaluation of Diabetic Peripheral Neuropathy [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(1): 1-6. |
| [9] | Guoying Zhang, MD, Ying Tang, BS, Mingyang Wang, MD, Weina Kong, MD. Diagnostic Utility of Superb Microvascular Imaging of ultrasound Examinations to Evaluate Hepatic Ischemia-reperfusion Injury [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(1): 15-21. |
| [10] | Hui Li, MD, Nan Zheng, MD, Penglin Zou, MD, Chao Jia, MD, Long Liu, MD, Gang Li, MD, Ziqi Wang, MD, Rong Wu, MD, Lianfang Du, MD, Qiusheng Shi, MD. The Role of Ultrasonography in the Diagnosis of Systemic Sarcoidosis: a Case Report and Literature Review [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(1): 32-38. |
| [11] | Yang Qi, MD, Dengsheng Sun, MD, Linyao Wang, MD, Jie Yu, MD, Ping Liang, MD. State-of-the-Art and Development Trend of Interventional Ultrasound in China [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 313-320. |
| [12] | Sebastián Eustaquio Martín Pérez, MSc, Raúl Hernández García, PT, Alberto Brito Lorenzo, PT, Carlos Daniel Sabater Cruz, PT, Mario Herrera Pérez, PhD, Fidel Rodríguez Hernández, PhD, Kristin Briem, PhD, Isidro Miguel Martín Pérez, MD. Ultrasonographic Identification of Muscle Atrophy in Hamstring Muscles after Anterior Cruciate Ligament Repair among Soccer Players: A Case-control Study [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 381-389. |
| [13] | Lujing Li, MD, Zuofeng Xu, MD. The Value of VTTQ Combined with B-mode US for Distinguishing Benign from Malignant Breast Masses by Comparing with SE: A Clinical Research [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 394-400. |
| [14] | Rodanthi Sfakiotaki, MS, Sergia Liasi, BM, Eleni Papaiakovou, BM, Irene Vraka, PhD, Marina Vakaki, PhD, Chrysoula Koumanidou, PhD. Juvenile Granulosa Cell Tumor of the Testis: A Preoperative Approach of the Diagnosis with Ultrasound [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 409-411. |
| [15] | Yiming Li, BM, Jing Xiao, MD, Fang Xie, MD, Yu Lin, BM, Mingbo Zhang, MD, Yukun Luo, MD. The Value of CEUS in the Diagnosis and Treatment of Thyroid Primary Squamous Cell Carcinoma: A Case Report [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 412-415. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Share: WeChat
Copyright ©2018 Advanced Ultrasound in Diagnosis and Therapy
|
 Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
