

ADVANCED ULTRASOUND IN DIAGNOSIS AND THERAPY >
Graphene Oxide/Polylactic Acid Microbubbles for Efficient Removal of Lead Ions from Aqueous Solution
Received date: 2021-09-24
Revised date: 2021-12-15
Accepted date: 2022-04-22
Online published: 2022-10-25
Objective: Heavy metal pollution has become one of the environmental contamination problems in today's world. Adsorption materials can effectively remove heavy metal ions from the water. There are some shortcomings for traditional adsorbents, such as difficult separation after adsorption, long separation time, and may cause secondary pollution in the environment without recycling. The aim of this study was to seek new materials with effective ways to absorb heavy metal ions in the water.
Methods: A new kind of adsorption material consisted of polylactic acid (PLA) microbubble and graphene oxide (GO) (i.e., PLA@GO microbubbles) was fabricated which can combine by electrostatic adsorption with the assistance of PAH. The influence of the initial concentration of heavy metal of lead ion, pH value, and absorption time on the adsorption effect of PLA@GO microbubbles was tested in this study.
Results: Graphene oxide modified on PLA microbubble has huge specific surface area and various functional groups, which can adsorb heavy metal ions in water. The resulting PLA@GO microbubble showed a homogeneous spherical structure with a size of 500-1.5 μm, which was suitable for its effective separation from water. The optimal dosage of PLA@GO microbubbles was 10 mg, the pH value of the solution was 5.0, and the adsorption time was 20 minutes which correlates to 75 mg/L of leadions.
Conclusion: The characteristics of the PLA@GO microbubbles showed a strong adsorption capacity, high adsorption efficiency, and a shorter balance time which provided an environment-friendly new material to remove heavy metal ions from water.
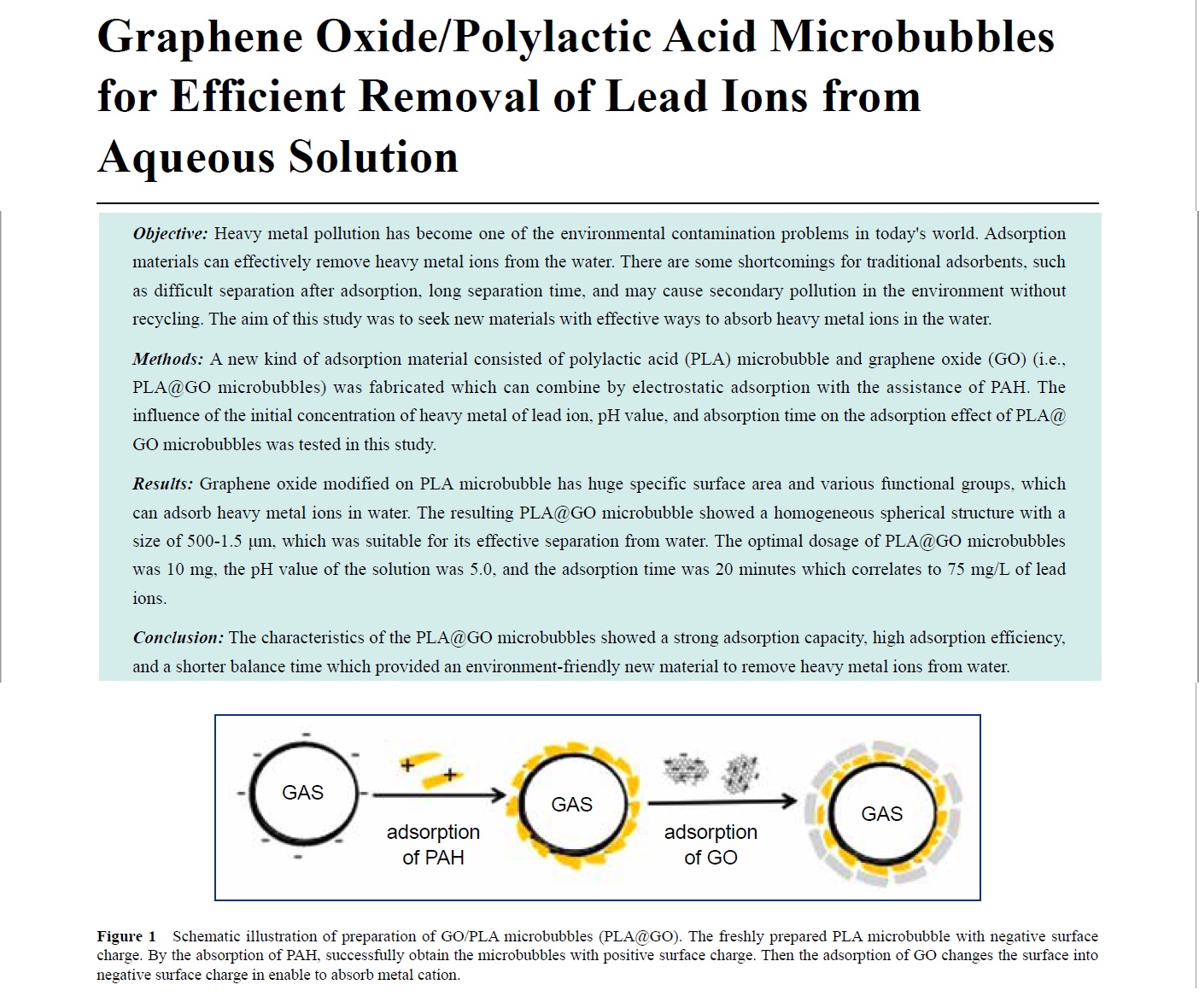
Key words: Graphene; Polylactic acid; Microbubble; Heavy metals; Lead
Meng Han, MD , Ruirui Kang, MD , Juanjuan Chen, MD . Graphene Oxide/Polylactic Acid Microbubbles for Efficient Removal of Lead Ions from Aqueous Solution[J]. ADVANCED ULTRASOUND IN DIAGNOSIS AND THERAPY, 2022 , 6(4) : 188 -194 . DOI: 10.37015/AUDT.2022.210030
| [1] | Fan L, Luo C, Lv Z, Lu F, Qiu H. Removal of Ag+ from water environment using a novel magnetic thiourea-chitosan imprinted Ag. J Hazard Mater 2011; 194:193-201. |
| [2] | Deng J, Wang Y, Liu X, Hu W, Zhu J, Zhu L. Spatial distribution and risk assessment of heavy metals and as pollution in the sediments of a shallow lak. Environ Monit Assess 2016; 188:296. |
| [3] | Khan A, Wang J, Li J, Wang X, Chen Z, Alsaedi A, et al. The role of graphene oxide and graphene oxide-based nanomaterials in the removal of pharmaceuticals from aqueous media: a revie. Environ Sci Pollut Res Int 2017; 24:7938-7958. |
| [4] | Simonovic SP. World water dynamics: global modeling of water resource. Journal of environmental management 2002; 66:249-267. |
| [5] | Ahmad SZN, Wan Salleh WN, Ismail AF, Yusof N, Mohd Yusop MZ, Aziz F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: toxicity, roles of functional groups and mechanism. Chemosphere 2020; 248:126008. |
| [6] | Liu J, Chen Y, Han T, Cheng M, Zhang W, Long J, et al. A biomimetic SiO2@chitosan composite as highly-efficient adsorbent for removing heavy metal ions in drinking wate. Chemosphere 2019; 214:738-742. |
| [7] | Jiang T, Dong M, Yan L, Fang M, Liu R. Removal of Cu (II) ions from aqueous solution using Fe/La-CTAB-Graphene oxide nanocomposite as efficient adsorben. J Nanosci Nanotechnol 2018; 18:4692-4699. |
| [8] | Park S, Lee KS, Bozoklu G, Cai W, Nguyen ST, Ruoff RS. Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linkin. ACS nano 2008; 2:572-578. |
| [9] | Huang Q, Chen Y, Yu H, Yan L, Zhang J, Wang B, et al. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: one-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chemical Engineering Journal 2018; 341:1-9. |
| [10] | Kong Q, Wei J, Hu Y, Wei C. Fabrication of terminal amino hyperbranched polymer modified graphene oxide and its prominent adsorption performance towards Cr (VI). J Hazard Mater 2019; 363:161-169. |
| [11] | Sitko R, Turek E, Zawisza B, Malicka E, Talik E, Heimann J, et al. Adsorption of divalent metal ions from aqueous solutions using graphene oxid. Dalton transactions (Cambridge, England: 2003) 2013; 42:5682-5689. |
| [12] | Wu Z, Deng W, Zhou W, Luo J. Novel magnetic polysaccharide/graphene oxide @Fe3O4 gel beads for adsorbing heavy metal ion. Carbohydr Polym 2019; 216:119-128. |
| [13] | Sharma VK, McDonald TJ, Kim H, Garg VK. Magnetic graphene-carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purificatio. Adv Colloid Interface Sci 2015; 225:229-240. |
| [14] | Singhvi MS, Zinjarde SS, Gokhale DV. Polylactic acid: synthesis and biomedical application. J Appl Microbiol 2019; 127:1612-1626. |
| [15] | Miao Z, Guo C, Li Z, Ke H, Dai Z. Fabrication of a multimodal microbubble platform for magnetic resonance, ultrasound and fluorescence imaging applicatio. J Nanosci Nanotechnol 2016; 16:2301-2306. |
| [16] | Feng S, Li X, Ma F, Liu R, Fu G, Xing S, et al. Prussian blue functionalized microcapsules for effective removal of cesium in water environment. Rsc Adv2016: 10. 1039. |
| [17] | Azmin M. Engineering ultrasound contrast agents for increased stability and nonlinearit. UCL (University College London); 2016. |
| [18] | Haeri SA, Abbasi S, Sajjadifar S. Bio-dispersive liquid liquid microextraction based on nano rhaminolipid aggregates combined with magnetic solid phase extraction using Fe3O4@PPy magnetic nanoparticles for the determination of methamphetamine in human urin. J Chromatogr B Analyt Technol Biomed Life Sci 2017; 1063:101-106. |
| [19] | Yang G, Xiao Z, Tang C, Deng Y, Huang H, He Z. Recent advances in biosensor for detection of lung cancer biomarker. Biosens Bioelectron 2019; 141:111416. |
| [20] | Yadav S, Goel N, Kumar V, Tikoo K, Singhal S. Removal of fluoroquinolone from aqueous solution using graphene oxide: experimental and computational elucidatio. Environ Sci Pollut Res Int 2018; 25:2942-2957. |
| [21] | Hadadian M, Goharshadi EK, Fard MM, Ahmadzadeh H. Synergistic effect of graphene nanosheets and zinc oxide nanoparticles for effective adsorption of Ni (II) ions from aqueous solution. Applied Physics A 2018; 124:239. |
| [22] | Liu X, Ma R, Wang X, Ma Y, Yang Y, Zhuang L, et al. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: a revie. Environ Pollut 2019; 252:62-73. |
| [23] | Ren Y, Zhang J, Guo J, Chen F, Yan F. Porous poly (ionic liquid) membranes as efficient and recyclable absorbents for heavy metal ions. Macromol Rapid Commun 2017; 38. |
/
| 〈 |
|
〉 |