

ADVANCED ULTRASOUND IN DIAGNOSIS AND THERAPY >
Advances in Targeted Tumor Diagnosis and Therapy Based on Ultrasound-Responsive Nanodroplets
Received date: 2020-05-06
Online published: 2020-09-29
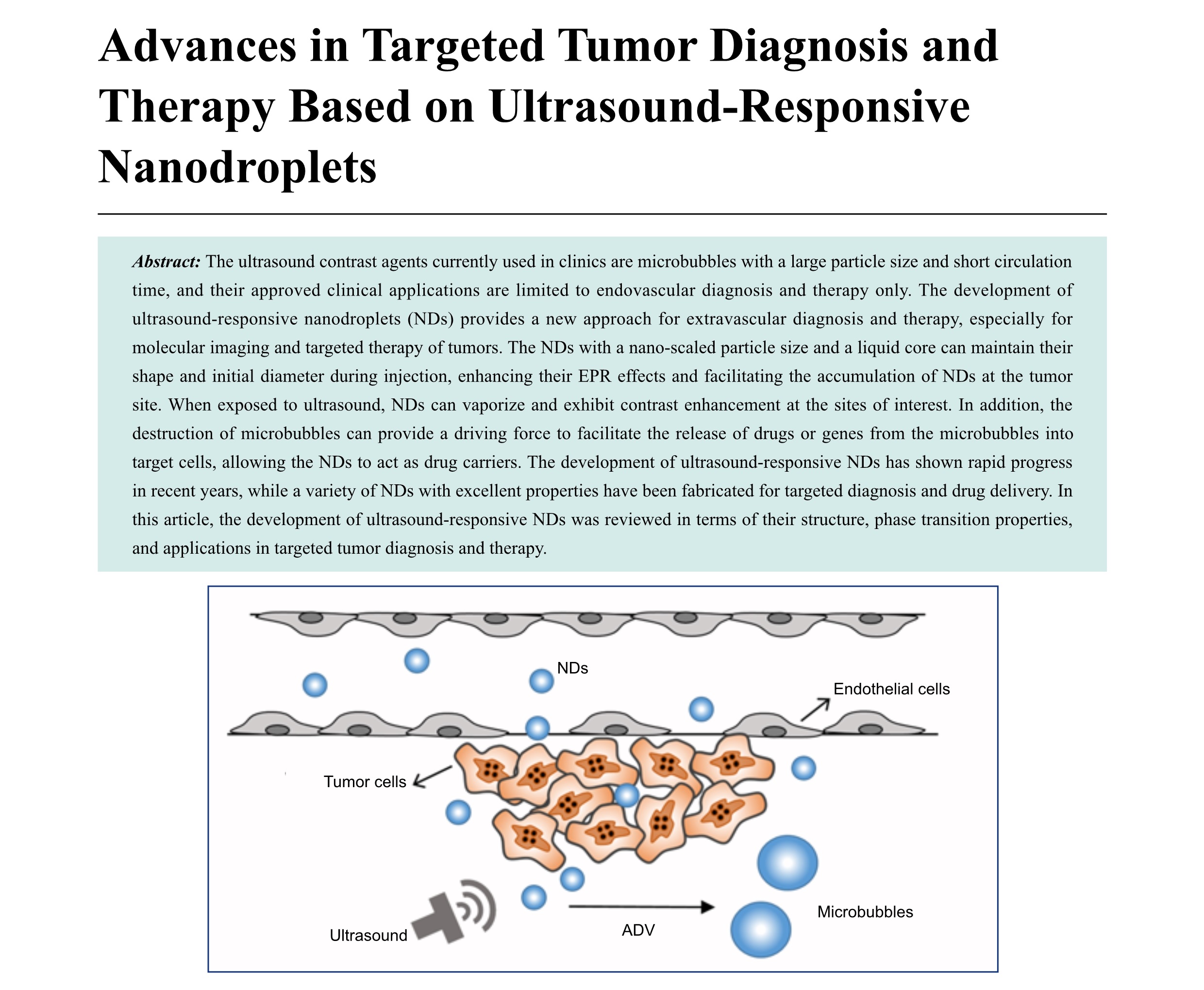
The ultrasound contrast agents currently used in clinics are microbubbles with a large particle size and short circulation time, and their approved clinical applications are limited to endovascular diagnosis and therapy only. The development of ultrasound-responsive nanodroplets (NDs) provides a new approach for extravascular diagnosis and therapy, especially for molecular imaging and targeted therapy of tumors. The NDs with a nano-scaled particle size and a liquid core can maintain their shape and initial diameter during injection, enhancing their EPR effects and facilitating the accumulation of NDs at the tumor site. When exposed to ultrasound, NDs can vaporize and exhibit contrast enhancement at the sites of interest. In addition, the destruction of microbubbles can provide a driving force to facilitate the release of drugs or genes from the microbubbles into target cells, allowing the NDs to act as drug carriers. The development of ultrasound-responsive NDs has shown rapid progress in recent years, while a variety of NDs with excellent properties have been fabricated for targeted diagnosis and drug delivery. In this article, the development of ultrasound-responsive NDs was reviewed in terms of their structure, phase transition properties, and applications in targeted tumor diagnosis and therapy.
Li, PhD Yaqiong , Liu, MD Ruiqing , Duan, MD Shaobo , Zhang, MD Lianzhong . Advances in Targeted Tumor Diagnosis and Therapy Based on Ultrasound-Responsive Nanodroplets[J]. ADVANCED ULTRASOUND IN DIAGNOSIS AND THERAPY, 2020 , 4(4) : 273 -283 . DOI: 10.37015/AUDT.2020.200043
| [1] | James ML, Gambhir SS. A molecular imaging primer: Modalities, imaging agents, and applications. Physio Rev 2012; 92:897-965. |
| [2] | Zlitni A, Gambhir SS. Molecular imaging agents for ultrasound. Curr Opin Chem Biol 2018; 45:113-120. |
| [3] | Güvener N, Appold L, de Lorenzi F, Golombek SK, Rizzo LY, Lammers T, et al. Recent Advances in Ultrasound-Based Diagnosis and Therapy with Micro- and Nanometer-Sized Formulations. Methods 2017; 130:4-13. |
| [4] | Lea-Banks H, O'Reilly MA, Hynynen K. Ultrasound-responsive droplets for therapy: A review. J Control Release 2019; 293:144-154. |
| [5] | Kee ALY, Teo BM. Biomedical applications of acoustically responsive phase shift nanodroplets: current status and future directions. Ultrason Sonochem 2019; 56:37-45. |
| [6] | Tang H, Zheng Y, Chen Y. Materials chemistry of nanoultrasonic biomedicine. Adv Mater 2017; 29:1604105. |
| [7] | Baghbani F, Chegeni M, Moztarzadeh F, Hadian-Ghazvini S, Raz M. Novel ultrasound-responsive chitosan/perfluorohexane nanodroplets for image-guided smart delivery of an anticancer agent: Curcumin. Mater Sci Eng C Mater Biol Appl 2017; 74:186-193. |
| [8] | Khadjavi A, Stura I, Prato M, Minero VG, Panariti A, Rivolta I, et al. ‘In vitro’, ‘in vivo’ and ‘in silico’ investigation of the anticancer effectiveness of oxygen-loaded chitosan-shelled nanodroplets as potential drug vector. Pharm Res 2018; 35:75. |
| [9] | Baghbani F, Chegeni M, Moztarzadeh F, Mohandesi JA, Mokhtari-Dizaji M. Ultrasonic nanotherapy of breast cancer using novel ultrasound-responsive alginate-shelled perfluorohexane nanodroplets: In vitro and in vivo evaluation. Mater Sci Eng C Mater Biol Appl 2017; 77:698-707. |
| [10] | Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics 2008; 48:260-270. |
| [11] | Rapoport N, Christensen DA, Kennedy AM, Nam K-H. Cavitation properties of block copolymer stabilized phase-shift nanoemulsions used as drug carriers. Ultrasound Med Biol 2010; 36:419-429. |
| [12] | Cao Y, Chen Y, Yu T, Guo Y, Liu F, Yao Y, et al. Drug release from phase-changeable nanodroplets triggered by low-intensity focused ultrasound. Theranostics 2018; 8:1327-39. |
| [13] | Mannaris C, Yang C, Carugo D, Owen J, Lee JY, Nwokeoha S, et al. Acoustically responsive polydopamine nanodroplets: a novel theranostic agent. Ultrason Sonochem 2020; 60:104782. |
| [14] | Kripfgans OD, Fabiilli ML, Carson PL, Fowlkes JB. On the acoustic vaporization of micrometer-sized droplets. J Acoust Soc Am 2004; 116:272-281. |
| [15] | Schad KC, Hynynen K. In Vitrocharacterization of perfluorocarbon droplets for focused ultrasound therapy. Phys Med Biol 2010; 55:4933-4947. |
| [16] | Kripfgans OD, Fowlkes JB, Miller DL, Eldevik OP, Carson PL. Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound Med Biol 2000; 26:1177-1189. |
| [17] | Haworth KJ, Fowlkes JB, Carson PL, Kripfgans OD. Towards aberration correction of transcranial ultrasound using acoustic droplet vaporization. Ultrasound Med Biol 2008; 34:435-445. |
| [18] | Gao J, Yu B, Li C, Xu M, Cao Z, Xie X, et al. Ultrasound triggered phase-change nanodroplets for doxorubicin prodrug delivery and ultrasound diagnosis: An in vitro study. Colloids Surf B Biointerfaces 2019; 174:416-425. |
| [19] | Dong W, Wu P, Zhou D, Huang J, Qin M, Yang X, et al. Ultrasound-mediated gene therapy of hepatocellular carcinoma using pre-microrna plasmid-loaded nanodroplets. Ultrasound Med Biol 2020; 46:90-107. |
| [20] | Rapoport NY, Efros AL, Christensen DA, Kennedy AM, Nam KH. Microbubble generation in phase-shift nanoemulsions used as anticancer drug carriers. Bubble Sci Eng Technol 2009; 1:31-39. |
| [21] | Rapoport N, Nam K-H, Gupta R, Gao Z, Mohan P, Payne A, et al. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J Control Release 2011; 153:4-15. |
| [22] | Yang Q, Li P, Ran H, Wan J, Chen H, Chen H, et al. Polypyrrole-coated phase-change liquid perfluorocarbon nanoparticles for the visualized photothermal-chemotherapy of breast cancer. Acta Biomater 2019; 90:337-349. |
| [23] | Phillips LC, Puett C, Sheeran PS, Dayton PA, Wilson Miller G, Matsunaga TO. Phase-shift perfluorocarbon agents enhance high intensity focused ultrasound thermal delivery with reduced near-field heating. J Acoust Soc Am 2013; 134:1473-82. |
| [24] | Sheeran PS, Wong VP, Luois S, McFarland RJ, Ross WD, Feingold S, et al. Decafluorobutane as a phase-change contrast agent for low-energy extravascular ultrasonic imaging. Ultrasound Med Biol 2011; 37:1518-1530. |
| [25] | Matsunaga T, Sheeran P, Luois S, Streeter J, Mullin L, Dayton P. Phase-change nanoparticles using highly volatile perfluorocarbons: toward a platform for extravascular ultrasound imaging. Theranostics 2012; 2:1185-98. |
| [26] | Li K, Liu Y, Zhang S, Xu Y, Jiang J, Yin F, et al. Folate receptor-targeted ultrasonic pfob nanoparticles: synthesis, characterization and application in tumor-targeted imaging. Int J Mol Med 2017; 39. |
| [27] | Sheeran PS, Yoo K, Williams R, Yin M, Foster FS, Burns PN. More than bubbles: Creating phase-shift droplets from commercially available ultrasound contrast agents. Ultrasound Med Biol 2017; 43:531-540. |
| [28] | Nakamura Y, Mochida A, Choyke PL, Kobayashi H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem 2016; 27:2225-38. |
| [29] | Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 2005; 338:284-293. |
| [30] | Zhang G, Lin S, Leow CH, Pang KT, Hernández-Gil J, Long NJ, et al. Quantification of vaporised targeted nanodroplets using high-frame-rate ultrasound and optics. Ultrasound Med Biol 2019; 45:1131-1142. |
| [31] | Hadinger KP, Marshalek JP, Sheeran PS, Dayton PA, Matsunaga TO. Optimization of phase-change contrast agents for targeting MDA-MB-231 breast cancer cells. Ultrasound Med Biol 2018; 44:2728-2738. |
| [32] | Liu J, Shang T, Wang F, Cao Y, Hao L, Ren J, et al. Low-intensity focused ultrasound (LIFU)-induced acoustic droplet vaporization in phase-transition perfluoropentane nanodroplets modified by folate for ultrasound molecular imaging. Int J Nanomedicine 2017; 12:911-923. |
| [33] | Gao D, Gao J, Xu M, Cao Z, Zhou L, Li Y, et al. Targeted Ultrasound-triggered phase transition nanodroplets for Her2-overexpressing breast cancer diagnosis and gene transfection. Mo Pharm 2017; 14:984-998. |
| [34] | Toumia Y, Cerroni B, Domenici F, Lange H, Bianchi L, Cociorb M, et al. Phase change ultrasound contrast agents with a photopolymerized diacetylene shell. Langmuir 2019; 35:10116-10127. |
| [35] | Lin CY, Pitt WG. Acoustic droplet vaporization in biology and medicine. Biomed Res Int 2013; 2013:404361. |
| [36] | Pitt WG, Singh RN, Perez KX, Husseini GA, Jack DR. Phase transitions of perfluorocarbon nanoemulsion induced with ultrasound: a mathematical model. Ultrason Sonochem 2014; 21:879-891. |
| [37] | Sheeran PS, Matsunaga TO, Dayton PA. Phase-transition thresholds and vaporization phenomena for ultrasound phase-change nanoemulsions assessed via high-speed optical microscopy. Phys Med Biol 2013; 58:4513-4534. |
| [38] | Paul SS, Paul AD. Phase-change contrast agents for imaging and therapy. Curr Pharm Des 2012; 18:2152-2165. |
| [39] | Kandadai MA, Mohan P, Lin G, Butterfield A, Skliar M, Magda JJ. Comparison of surfactants used to prepare aqueous perfluoropentane emulsions for pharmaceutical applications. Langmuir 2010; 26:4655-4660. |
| [40] | Kang ST, Yeh CK. Intracellular acoustic droplet vaporization in a single peritoneal macrophage for drug delivery applications. Langmuir 2011; 27:13183-13188. |
| [41] | Shpak O, Verweij M, Vos HJ, de Jong N, Lohse D, Versluis M. Acoustic droplet vaporization is initiated by superharmonic focusing. Proc Natl Acad Sci USA 2014; 111:1697-1702. |
| [42] | Reznik N, Lajoinie G, Shpak O, Gelderblom EC, Williams R, de Jong N, et al. On the acoustic properties of vaporized submicron perfluorocarbon droplets. Ultrasound Med Biol 2014; 40:1379-1384. |
| [43] | Evans DR, Parsons DF, Craig VS. Physical properties of phase-change emulsions. Langmuir 2006; 22:9538-9545. |
| [44] | Fabiilli ML, Haworth KJ, Fakhri NH, Kripfgans OD, Carson PL, Fowlkes JB. The role of inertial cavitation in acoustic droplet vaporization. IEEE Trans Ultrason Ferroelectr Freq Control 2009; 56:1006-1017. |
| [45] | Giesecke T, Hynynen K. Ultrasound-mediated cavitation thresholds of liquid perfluorocarbon droplets in vitro. Ultrasound Med Biol 2003; 29:1359-1365. |
| [46] | Lo AH, Kripfgans OD, Carson PL, Rothman ED, Fowlkes JB. Acoustic droplet vaporization threshold: effects of pulse duration and contrast agent. IEEE Trans Ultrason Ferroelectr Freq Control 2007; 54:933-46. |
| [47] | Wischhusen J, Padilla F. Ultrasound-targeted microbubble destruction (UTMD) for localized drug delivery into tumor tissue. IRBM 2019; 40:10-15. |
| [48] | Abou-Elkacem L, Bachawal SV, Willmann JK. Ultrasound molecular imaging: moving toward clinical translation. Eur J Radiol 2015; 84:1685-1693. |
| [49] | Kiessling F, Fokong S, Bzyl J, Lederle W, Palmowski M, Lammers T. Recent advances in molecular, multimodal and theranostic ultrasound imaging. Adv Drug Deliv Rev 2014; 72:15-27. |
| [50] | Williams R, Wright C, Cherin E, Reznik N, Lee M, Gorelikov I, et al. Characterization of submicron phase-change perfluorocarbon droplets for extravascular ultrasound imaging of cancer. Ultrasound Med Biol 2013; 39:475-89. |
| [51] | Choi D, Jeon S, You D, Um W, Kim JY, Yoon H, et al. Iodinated echogenic glycol chitosan nanoparticles for x-ray CT/US dual imaging of tumor. Nanotheranostics 2018; 2:117-27. |
| [52] | Dimcevski G, Kotopoulis S, Bj?nes T, Hoem D, Schj?tt J, Gjertsen BT, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release 2016; 243:172-181. |
| [53] | Luo W, Wen G, Yang L, Tang J, Wang J, Wang J, et al. Dual-targeted and pH-sensitive doxorubicin prodrug-microbubble complex with ultrasound for tumor treatment. Theranostics 2017; 7:452-65. |
| [54] | Yan F, Li L, Deng Z, Jin Q, Chen J, Yang W, et al. Paclitaxel-liposome-microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J Control Release 2013; 166:246-55. |
| [55] | Tinkov S, Coester C, Serba S, Geis NA, Katus HA, Winter G, et al. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Control Release 2010; 148:368-72. |
| [56] | Ho YJ, Chiang YJ, Kang ST, Fan CH, Yeh CK. Camptothecin-loaded fusogenic nanodroplets as ultrasound theranostic agent in stem cell-mediated drug-delivery system. J Control Release 2018; 278:100-109. |
| [57] | Zhao YZ, Zhang M, Wong HL, Tian XQ, Zheng L, Yu XC, et al. Prevent diabetic cardiomyopathy in diabetic rats by combined therapy of aFGF-loaded nanoparticles and ultrasound-targeted microbubble destruction technique. J Control Release 2016; 223 : 11-21. |
| [58] | Paproski RJ, Forbrich A, Hitt M, Zemp R. RNA biomarker release with ultrasound and phase-change nanodroplets. Ultrasound Med Biol 2014; 40:1847-56. |
| [59] | Gao D, Xu M, Cao Z, Gao J, Chen Y, Li Y, et al. Ultrasound-triggered phase-transition cationic nanodroplets for enhanced gene delivery. ACS Appl Mater Interfaces 2015; 7:13524-13537. |
| [60] | Guo H, Xu M, Cao Z, Li W, Chen L, Xie X, et al. Ultrasound-assisted miR-122-loaded polymeric nanodroplets for hepatocellular carcinoma gene therapy. Mo Pharm 2020; 17:541-553. |
| [61] | Zhao Y, Song W, Wang D, Ran H, Wang R, Yao Y, et al. Phase-shifted PFH@PLGA/Fe3O4 nanocapsules for MRI/US imaging and photothermal therapy with near-infrared irradiation. ACS Appl Mater Interfaces 2015; 7:14231-14242. |
| [62] | Zhang L, Yi H, Song J, Huang J, Yang K, Tan B, et al. Mitochondria-targeted and ultrasound-activated nanodroplets for enhanced deep-penetration sonodynamic cancer therapy. ACS Appl Mater Interfaces 2019; 11:9355-9366. |
| [63] | Chang N, Qin D, Wu P, Xu S, Wang S, Wan M. IR780 loaded perfluorohexane nanodroplets for efficient sonodynamic effect induced by short-pulsed focused ultrasound. Ultrason Sonochem 2019; 53:59-67. |
/
| 〈 |
|
〉 |