Advanced Ultrasound in Diagnosis and Therapy ›› 2025, Vol. 9 ›› Issue (2): 171-180.doi: 10.37015/AUDT.2025.240074
• Original Research • Previous Articles Next Articles
Xiang Hongjina, Huang Linb, Zheng Zhuc, Li Jiawua, Qiu Tingtinga, Wu Zhenrud, Shi Yujund, Jiang Huabeie, Ling Wenwua,*( ), Luo Yana,*(
), Luo Yana,*( )
)
Received:2025-02-27
Revised:2024-12-22
Accepted:2025-05-21
Online:2025-06-30
Published:2025-07-06
Contact:
Department of Ultrasound, West China Hospital, Sichuan University, 37 Guoxue Alley, Chengdu, Sichuan, China. e-mail: Xiang Hongjin, Huang Lin, Zheng Zhu, Li Jiawu, Qiu Tingting, Wu Zhenru, Shi Yujun, Jiang Huabei, Ling Wenwu, Luo Yan. Evaluation of Hepatic Steatosis Grades with Thermoacoustic Imaging in a Rabbit Model. Advanced Ultrasound in Diagnosis and Therapy, 2025, 9(2): 171-180.
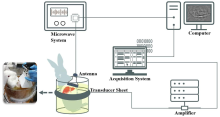
Figure 1
Schematic of the in-vivo liver thermoacoustic imaging system. A customized microwave generator irradiated the upper abdomens of the rabbits through a standard horn antenna. Thermoacoustic (TA) signal was captured by transducer sheets and then collected by the acquisition system. The whole imaging procedure was controlled by a personal computer, allowing the TA image to be produced every 2 seconds."
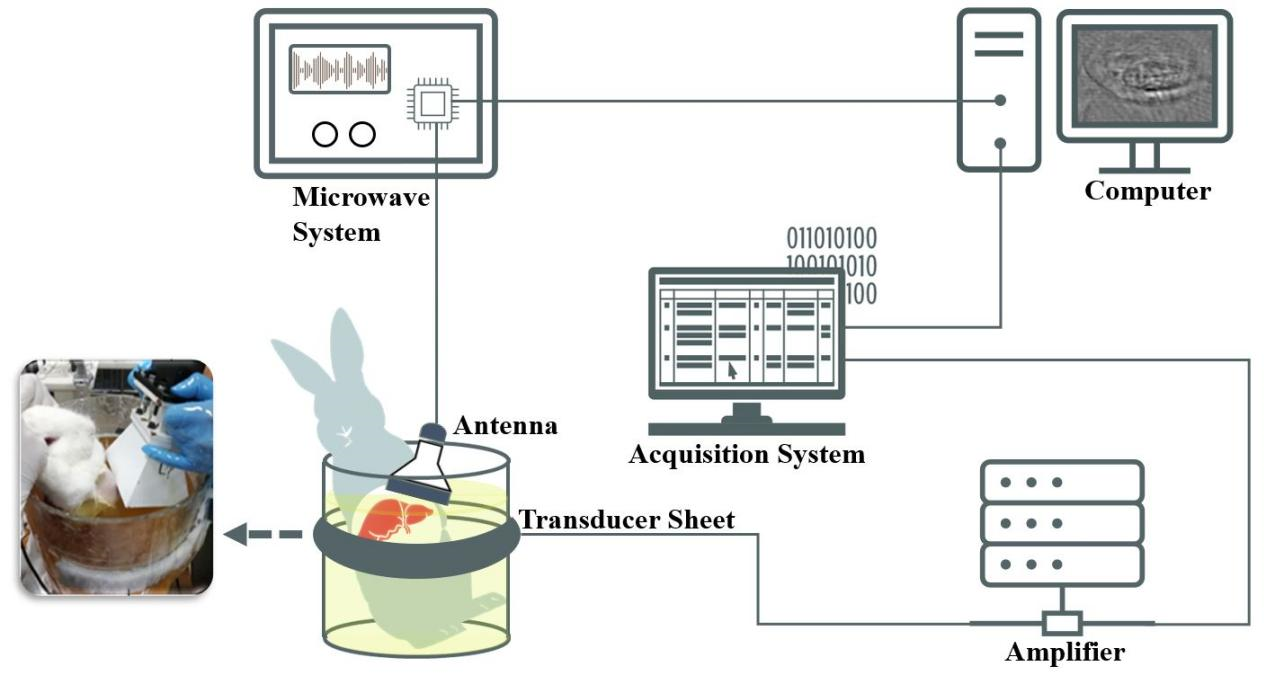
Figure 2
Thermoacoustic imaging (TAI) and histopathologic evaluation for the different steatosis grades. (A) Raw thermoacoustic (TA) images; (B) the liver region extracted from the TA image. The bar represents the grayscale for (A) & (B); (C) the livers were freed after an in-vivo TAI study; (D) photomicrograph (hematoxylin-eosin stain; original magnification, × 400); (E) photomicrograph (oil red stain; original magnification, × 400). S0, no steatosis; S1, mild steatosis; S2, moderate steatosis; S3, severe steatosis."
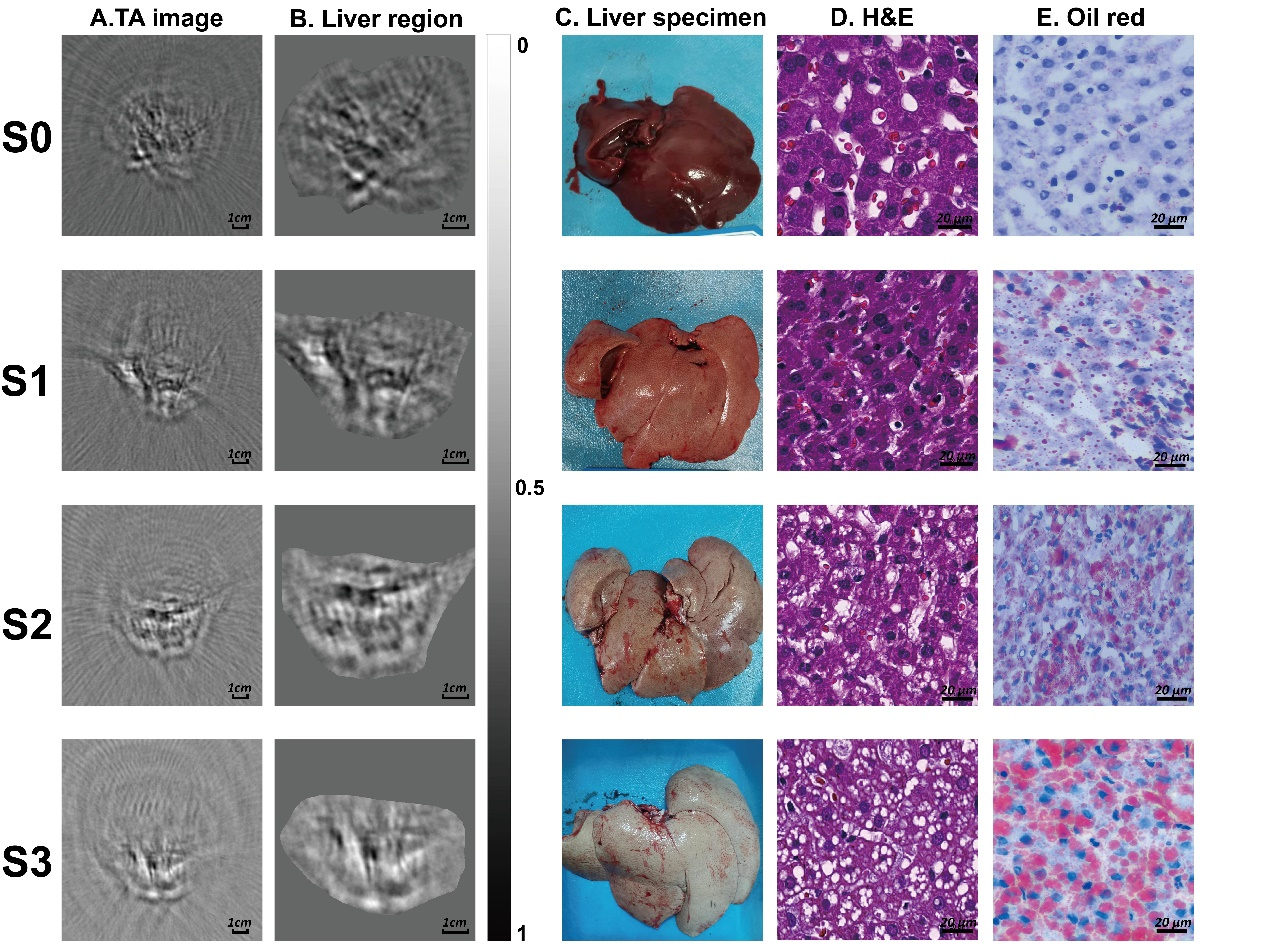
Table 1
The result of hepatic steatosis grades and thermoacoustic fat coefficients"
| Parameter | Hepatic steatosisb | |||
|---|---|---|---|---|
| S0 | S1 | S2 | S3 | |
| Number | 4 | 16 | 6 | 7 |
| mean/max (×10-3)a | 2.21 (1.95-2.32) | 2.52 (2.25-2.86) | 3.74 (3.31-4.49) | 5.18 (4.91-5.95) |
| mean/std (×10-2)a | 2.62 (2.36-2.99) | 2.63 (2.32-3.16) | 3.68 (3.23-4.49) | 5.18 (4.84-5.39) |
Table 2
Pathologic features of each rabbit"
| Rabbit No. | Fat percentage (%) | Steatosisa | Ballooninga | Lobular inflammationa | Fibrosisa |
|---|---|---|---|---|---|
| 1 | 0.00 | 0 | 0 | 0 | 0 |
| 2 | 0.00 | 0 | 0 | 0 | 0 |
| 3 | 4.00 | 0 | 0 | 0 | 0 |
| 4 | 4.77 | 0 | 0 | 0 | 0 |
| 5 | 13.43 | 1 | 2 | 0 | 0 |
| 6 | 16.45 | 1 | 0 | 0 | 0 |
| 7 | 18.04 | 1 | 0 | 0 | 1 |
| 8 | 20.00 | 1 | 1 | 0 | 0 |
| 9 | 20.00 | 1 | 2 | 1 | 2 |
| 10 | 21.32 | 1 | 0 | 0 | 1 |
| 11 | 22.55 | 1 | 1 | 0 | 2 |
| 12 | 24.00 | 1 | 2 | 0 | 0 |
| 13 | 25.00 | 1 | 0 | 0 | 0 |
| 14 | 25.20 | 1 | 2 | 1 | 1 |
| 15 | 27.34 | 1 | 1 | 1 | 2 |
| 16 | 28.95 | 1 | 0 | 0 | 1 |
| 17 | 30.00 | 1 | 1 | 0 | 1 |
| 18 | 30.00 | 1 | 2 | 0 | 1 |
| 19 | 30.51 | 1 | 0 | 0 | 0 |
| 20 | 33.00 | 1 | 2 | 2 | 3 |
| 21 | 35.00 | 2 | 1 | 0 | 1 |
| 22 | 36.40 | 2 | 0 | 2 | 1 |
| 23 | 43.00 | 2 | 1 | 0 | 0 |
| 24 | 50.00 | 2 | 2 | 1 | 4 |
| 25 | 60.00 | 2 | 2 | 0 | 1 |
| 26 | 60.00 | 2 | 1 | 1 | 3 |
| 27 | 68.00 | 3 | 2 | 1 | 4 |
| 28 | 70.00 | 3 | 1 | 0 | 1 |
| 29 | 79.00 | 3 | 2 | 2 | 2 |
| 30 | 80.00 | 3 | 1 | 0 | 0 |
| 31 | 80.00 | 3 | 1 | 1 | 4 |
| 32 | 80.00 | 3 | 2 | 2 | 2 |
| 33 | 90.00 | 3 | 1 | 2 | 0 |
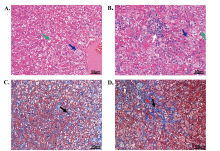
Figure 3
The pathologic features of two rabbits. (A-B) Hematoxylin-eosin stain of two rabbits. (C-D) The corresponding Masson stain of these two rabbits. Scalar bars = 50 μm. Green arrow, steatosis; Blue arrow, ballooning hepatocyte; White arrow, lobular inflammation; Black arrow, fibrosis."
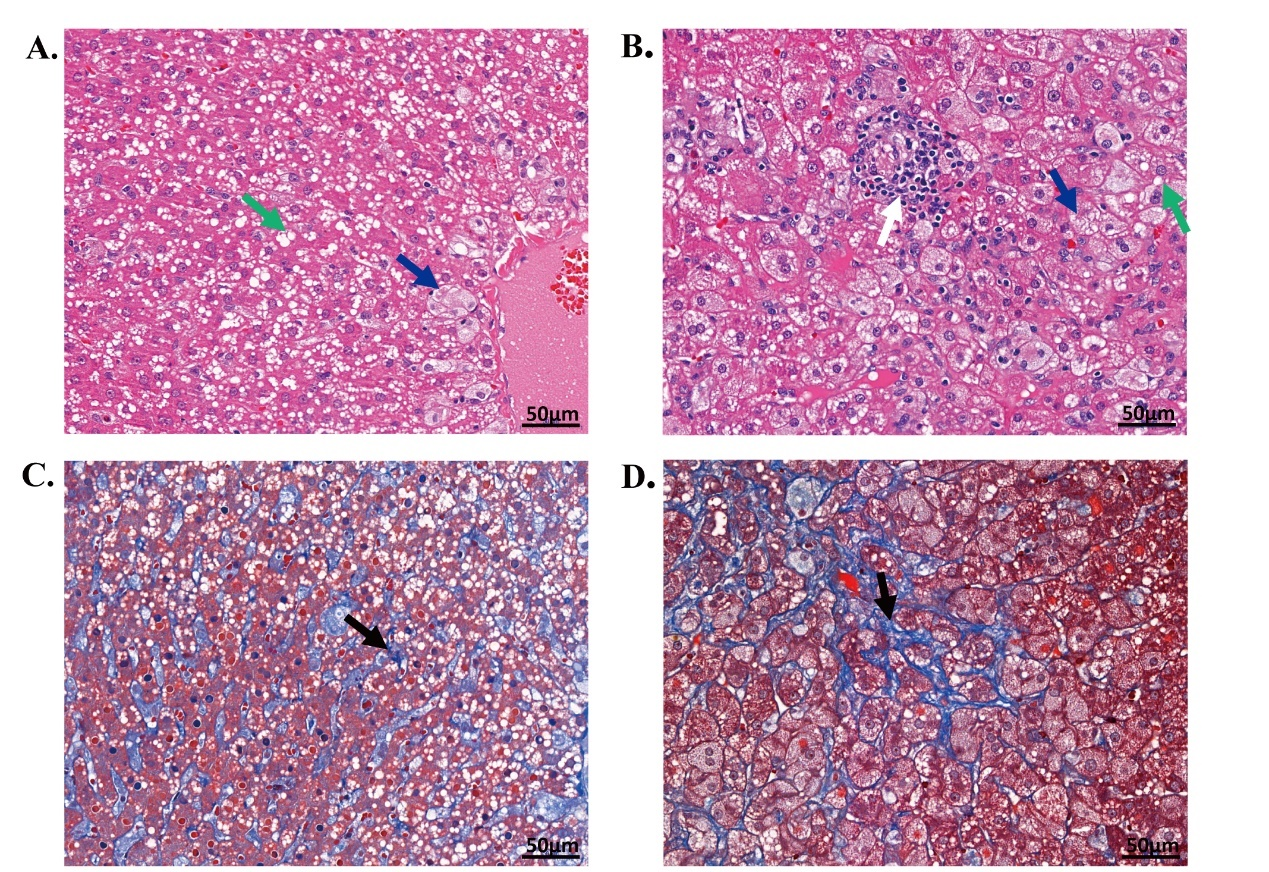
Figure 4
The correlations between the thermoacoustic fat coefficients (A, mean/max; B, mean/std) and the liver fat percentage determined by pathologic examinations. Pearson rho is the Pearson coefficient between the thermoacoustic fat coefficient and the fat percentage. Mean, max, and std are the mean value, the maximum, and the standard deviation of the thermoacoustic signal, respectively."
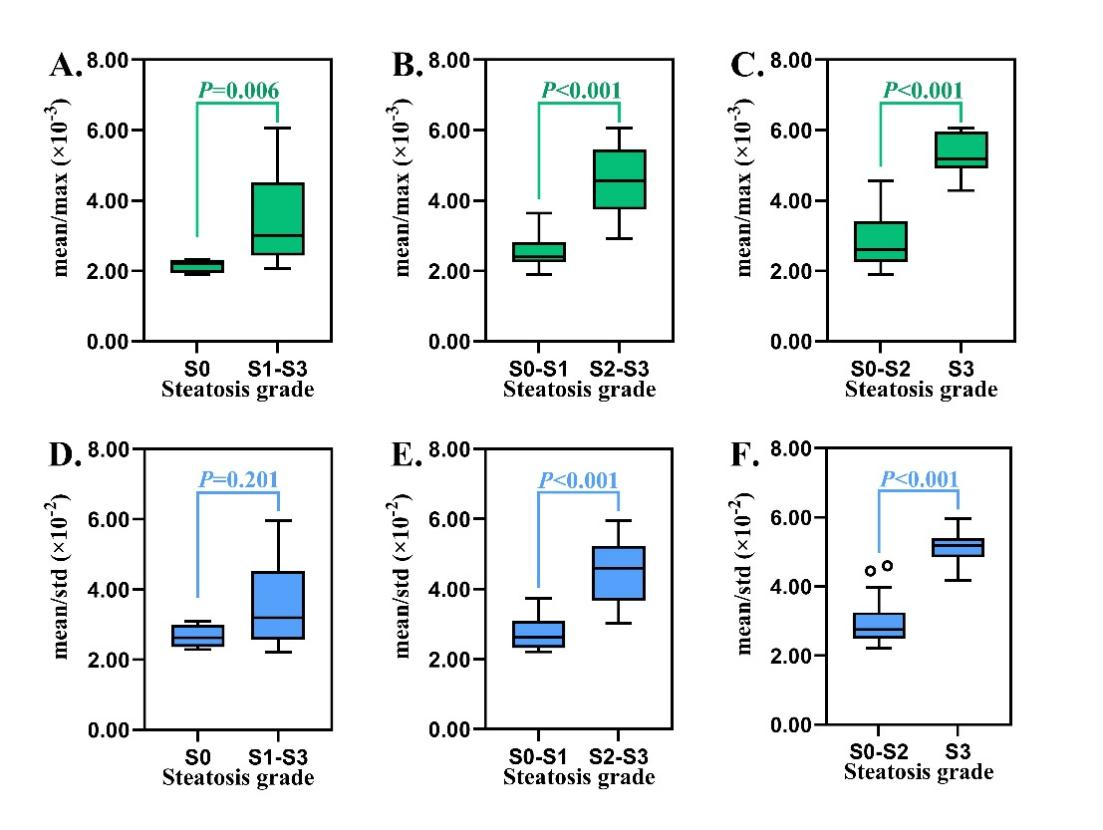
Table 3
Diagnostic performance of the mean/max and mean/std derived from thermoacoustic data"
| Aim | Cutoff | AUCa | Sen (%)b | Spe (%)b | PPV (%)b | NPV (%)b |
|---|---|---|---|---|---|---|
| mean/max | ||||||
| ≥ S1 | 2.33×10-3 | 0.905 (0.791, 1.000) | 83 (24/29) | 100 (4/4) | 100 (24/24) | 44 (4/9) |
| ≥ S2 | 3.40×10-3 | 0.981 (0.945, 1.000) | 92 (12/13) | 95 (19/20) | 92 (12/13) | 95 (19/20) |
| ≥ S3 | 3.99×10-3 | 0.989 (0.961, 1.000) | 100 (7/7) | 92 (24/26) | 78 (7/9) | 100 (24/24) |
| mean/std | ||||||
| ≥ S1 | 3.10×10-2 | 0.724 (0.532, 0.916) | 55 (16/29) | 100 (4/4) | 100 (16/16) | 24 (4/17) |
| ≥ S2 | 3.23×10-2 | 0.969 (0.916, 1.000) | 92 (12/13) | 85 (17/20) | 80 (12/15) | 94 (17/18) |
| ≥ S3 | 3.96×10-2 | 0.989 (0.961, 1.000) | 100 (7/7) | 92 (24/26) | 78 (7/9) | 100 (24/24) |
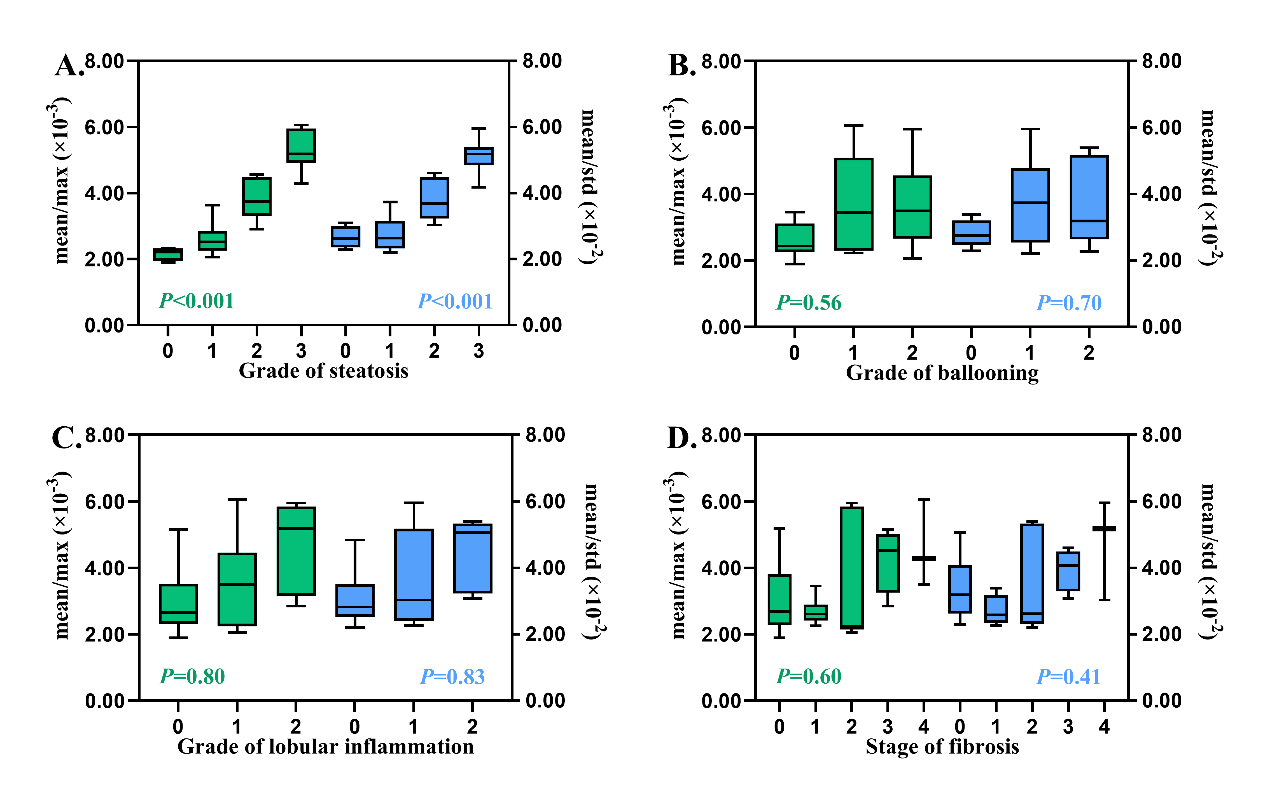
Figure 7
The box plots of the mean/max and mean/std derived from thermoacoustic imaging according to (A) grade of steatosis, (B) grade of ballooning, (C) grade of lobular inflammation and (D) stage of fibrosis. The left boxes (in green) and the left y-axis represent the mean/max, while the right boxes (in blue) and the right y-axis represent the mean/std. P values in green and blue were derived from the multivariate linear regression analysis for mean/max and mean/std, respectively. Mean, max, and std are the mean value, the maximum, and the standard deviation of the thermoacoustic signals, respectively."
| [1] | Powell EE, Wong VWS, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021;397:2212-2224. |
| [2] | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-357. |
| [3] | Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 2021;70:1375-1382. |
| [4] | Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol 2022;18:55-66. |
| [5] | Tilg H, Targher G. NAFLD-related mortality: simple hepatic steatosis is not as 'benign' as thought. Gut 2021;70:1212-1213. |
| [6] | Suliman I, Abdelgelil N, Kassamali F, Hassanein TI. The effects of hepatic steatosis on the natural history of HBV infection. Clin Liver Dis 2019;23:433-450. |
| [7] | Gordon A, McLean CA, Pedersen JS, Bailey MJ, Roberts SK. Hepatic steatosis in chronic hepatitis B and C: Predictors, distribution and effect on fibrosis. J Hepatol 2005;43:38-44. |
| [8] | Bedossa P, the FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014;60:565-575. |
| [9] | Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol 2018;24:3361-3373. |
| [10] | Ferraioli G, Berzigotti A, Barr RG, Choi BI, Cui XW, Dong Y, et al. Quantification of liver fat content with ultrasound: a WFUMB position paper. Ultrasound Med Biol 2021;47:2803-2820. |
| [11] | Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of liver fat content with CT and MRI: state of the art. Radiology 2021;301:250-262. |
| [12] | Zhang L, Qin H, Zeng F, Wu Z, Wu L, Zhao S, et al. A stimulated liquid-gas phase transition nanoprobe dedicated to enhance the microwave thermoacoustic imaging contrast of breast tumors. Nanoscale 2020;12:16034-16040. |
| [13] | Li J, Wang B, Zhang D, Li C, Zhu Y, Zou Y, et al. A preclinical system prototype for focused microwave breast hyperthermia guided by compressive thermoacoustic tomography. IEEE Trans Biomed Eng 2021;68:2289-2300. |
| [14] | Yan A, Lin L, Liu C, Shi J, Na S, Wang LV. Microwave-induced thermoacoustic tomography through an adult human skull. Med Phys 2019;46:1793-1797. |
| [15] | Chi Z, Zhao Y, Yang J, Li T, Zhang G, Jiang H. Thermoacoustic tomography of in vivo human finger joints. IEEE Trans Biomed Eng 2019;66:1598-1608. |
| [16] | Kruger RA, Miller KD, Reynolds HE, Kiser WL, Reinecke DR, Kruger GA. Breast cancer in vivo: contrast enhancement with thermoacoustic CT at 434 MHz—feasibility study. Radiology 2000;216:279-283. |
| [17] | C G.Compilation of the dielectric properties of body tissues at RF and microwave frequencies. Texas: Air Force materiel command Brooks Air Force Base 1996. |
| [18] | Van Herck M, Vonghia L, Francque S. Animal models of nonalcoholic fatty liver disease-a starter's guide. Nutrients 2017;9:1072. |
| [19] | Xiang H, Zheng Z, Huang L, Qiu T, Luo Y, Jiang H. In vivo liver thermoacoustic imaging and demonstration based on localization wire. Med Phys 2021;48:1608-1615. |
| [20] | IEEE-SA Standards Board. IEEE standard for safety levels with respect to human exposure to radio frequency electromagnetic fields, 3 kHz to 300 GHz. IEEE Std C95.1-2005 (Revision of IEEE Std C95.1-1991) 2006;pp1-238. |
| [21] | Chen YS, Yoon SJ, Frey W, Dockery M, Emelianov S.Dynamic contrast-enhanced photoacoustic imaging using photothermal stimuli-responsive composite nanomodulators. Nat Commun 2017;8:15782. |
| [22] | Davis MI, Chute DF, Chung RT, Sise ME.When and how can nephrologists treat hepatitis C virus infection in dialysis patients? Semin Dial 2018;31:26-36. |
| [23] | Luo Y, Abiri P, Zhang S, Chang CC, Kaboodrangi AH, Li R, et al. Non-invasive electrical impedance tomography for multi-scale detection of liver fat content. Theranostics 2018;8:1636-1647. |
| [24] | Bohte AE, van Werven JR, Bipat S, Stoker J.The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 2011;21:87-97. |
| [25] | Wang HW, Chai N, Wang P, Hu S, Dou W, Umulis D, et al. Label-free bond-selective imaging by listening to vibrationally excited molecules. Phys Rev Lett 2011;106:238106. |
| [26] | Xu G, Meng ZX, Lin JD, Yuan J, Carson PL, Joshi B, et al. The functional pitch of an organ: quantification of tissue texture with photoacoustic spectrum analysis. Radiology 2014;271:248-254. |
| [27] | Chang CC, Huang ZY, Shih SF, Luo Y, Ko A, Cui Q, et al. Electrical impedance tomography for non-invasive identification of fatty liver infiltrate in overweight individuals. Sci Rep 2021;11:19859. |
| [28] | Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 2018;68:763-772. |
| [29] | Lee DH, Lee JY, Lee KB, Han JK. Evaluation of hepatic steatosis by using acoustic structure quantification us in a rat model: comparison with pathologic examination and MR spectroscopy. Radiology 2017;285:445-453. |
| [30] | Mercep E, Dean-Ben XL, Razansky D. Combined pulse-echo ultrasound and multispectral optoacoustic tomography with a multi-segment detector array. IEEE Trans Med Imaging 2017;36:2129-2137. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Share: WeChat
Copyright ©2018 Advanced Ultrasound in Diagnosis and Therapy
|
 Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
