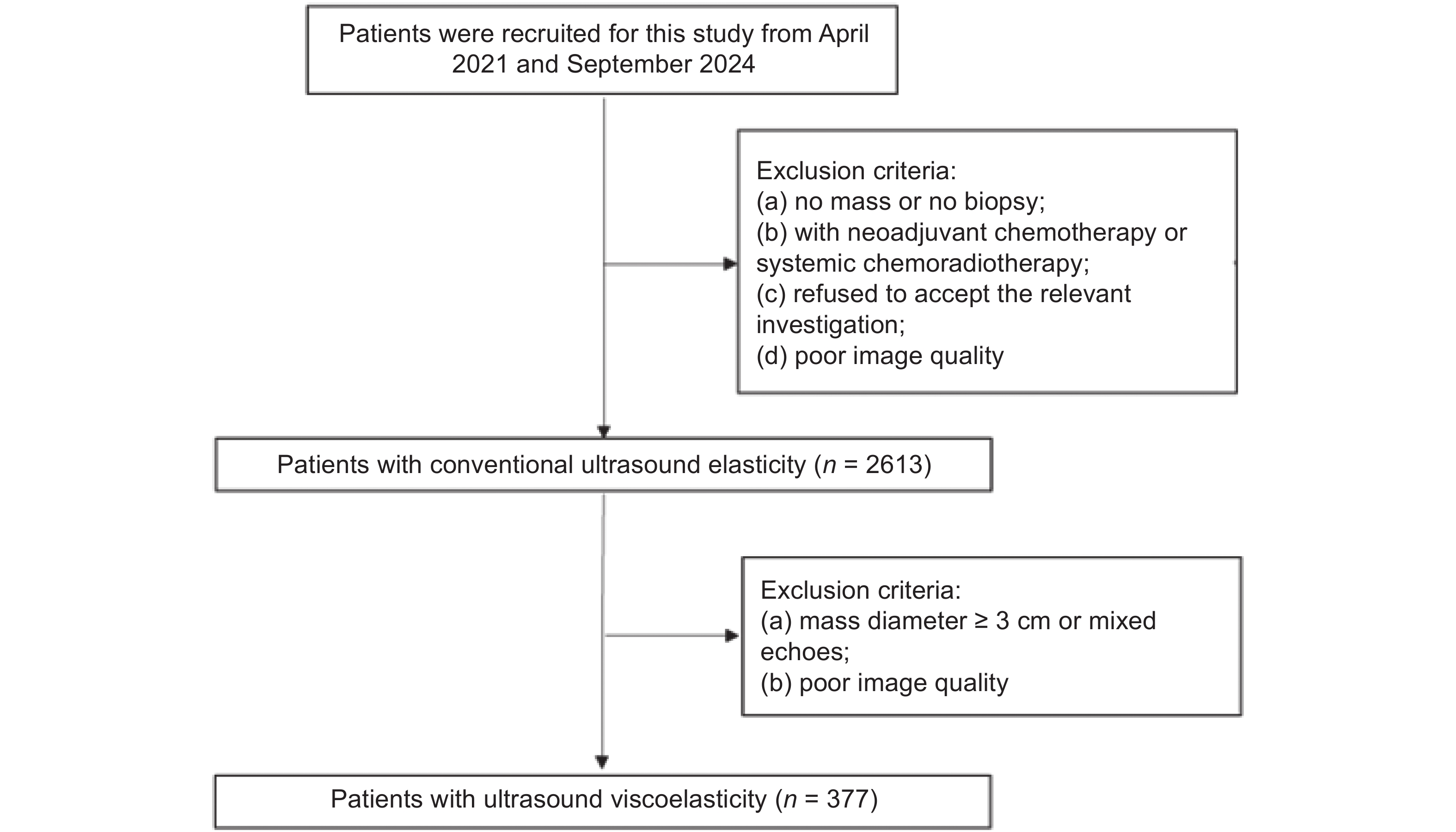
| [1] |
Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol 2022; 95: 20211033.
|
| [2] |
Zhang L, Dong YJ, Zhou JQ, Jia XH, Li S, Zhan WW. Similar reproducibility for strain and shear wave elastography inbreast mass evaluation: a prospective study using the same ultrasound system. Ultrasound Med Biol 2020; 46: 981-991.
|
| [3] |
Barr RG, Nakashima K, Amy D, Cosgrove D, Farrokh A, Schafer F, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: breast. Ultrasound Med Biol 2015; 41: 1148-1160.
|
| [4] |
Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 2015; 41: 1126-1147.
|
| [5] |
Zheng X, Li F, Xuan ZD, Wang Y, Zhang L. Combination of shear wave elastography and BI-RADS in identification of solid breast masses. BMC Med Imaging 2021; 21: 183.
|
| [6] |
Dong M, Xing B, Zhang B, Xu X, Zhou Q. Diagnostic performance and accuracy of strain elastography for BI-RADS category 4 lesions among Asian females. J Coll Physicians Surg Pak 2023; 33: 1181-1187.
|
| [7] |
Rus G, Faris IH, Torres J, Callejas A, Melchor J. Why are viscosity and nonlinearity bound to make an impact in clinical elastographic diagnosis? Sensors (Basel) 2020; 20.
|
| [8] |
Bhatt M, Moussu MAC, Chayer B, Destrempes F, Gesnik M, Allard L, et al. Reconstruction of viscosity maps in ultrasound shear wave elastography. IEEE Trans Ultrason Ferroelectr Freq Control 2019.
|
| [9] |
Kumar V, Denis M, Gregory A, Bayat M, Mehrmohammadi M, Fazzio R, et al. Viscoelastic parameters as discriminators of breast masses: initial human study results. PLoS One 2018; 13: e0205717.
|
| [10] |
Jia W, Xia S, Jia X, Tang B, Cheng S, Nie M, et al. Ultrasound viscosity imaging in breast lesions: a multicenter prospective study. Acad Radiol 2024; 31: 3499-3510.
|
| [11] |
Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 2012; 262: 435-449.
|
| [12] |
Zhang L, Xu J, Wu H, Liang W, Ye X, Tian H, et al. Screening breast lesions using shear modulus and its 1-mm shell in sound touch elastography. Ultrasound Med Biol 2019; 45: 710-719.
|
| [13] |
Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer 2003; 3: 921-930.
|
| [14] |
Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 2006; 4: 38.
|
| [15] |
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139: 891-906.
|
| [16] |
Wang ZL, Sun L, Li Y, Li N. Relationship between elasticity and collagen fiber content in breast disease: a preliminary report. Ultrasonics 2015; 57: 44-49.
|
| [17] |
Soby L, Jamieson AM, Blackwell J, Choi HU, Rosenberg LC. Viscoelastic and rheological properties of concentrated solutions of proteoglycan subunit and proteoglycan aggregate. Biopolymers 1990; 29: 1587-1592.
|
| [18] |
Karousou E, D'Angelo ML, Kouvidi K, Vigetti D, Viola M, Nikitovic D, et al. Collagen VI and hyaluronan: the common role in breast cancer. Biomed Res Int 2014; 2014: 606458.
|
| [19] |
Chan CJ, Costanzo M, Ruiz-Herrero T, Mönke G, Petrie RJ, Bergert M, et al. Hydraulic control of mammalian embryo size and cell fate. Nature 2019; 571: 112-116.
|
| [20] |
Nelson CM, Gleghorn JP, Pang MF, Jaslove JM, Goodwin K, Varner VD, et al. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 2017; 144: 4328-4335.
|
| [21] |
Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ. Bending gradients: how the intestinal stem cell gets its home. Cell 2015; 161: 569-580.
|
| [22] |
Harris AR, Peter L, Bellis J, Baum B, Kabla AJ, Charras GT. Characterizing the mechanics of cultured cell monolayers. Proc Natl Acad Sci U S A 2012; 109: 16449-16454.
|
| [23] |
Bera K, Kiepas A, Godet I, Li Y, Mehta P, Ifemembi B, et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature 2022; 611: 365-373.
|
| [24] |
Elosegui-Artola A, Gupta A, Najibi AJ, Seo BR, Garry R, Tringides CM, et al. Matrix viscoelasticity controls spatiotemporal tissue organization. Nat Mater 2023; 22: 117-127.
|
| [25] |
Morla-Barcelo PM, Laguna-Macarrilla D, Cordoba O, Matheu G, Oliver J, Roca P, et al. Unraveling malignant phenotype of peritumoral tissue: transcriptomic insights into early-stage breast cancer. Breast Cancer Res 2024; 26: 89.
|
| [26] |
Barr RG. Future of breast elastography. Ultrasonography 2019; 38: 93-105.
|