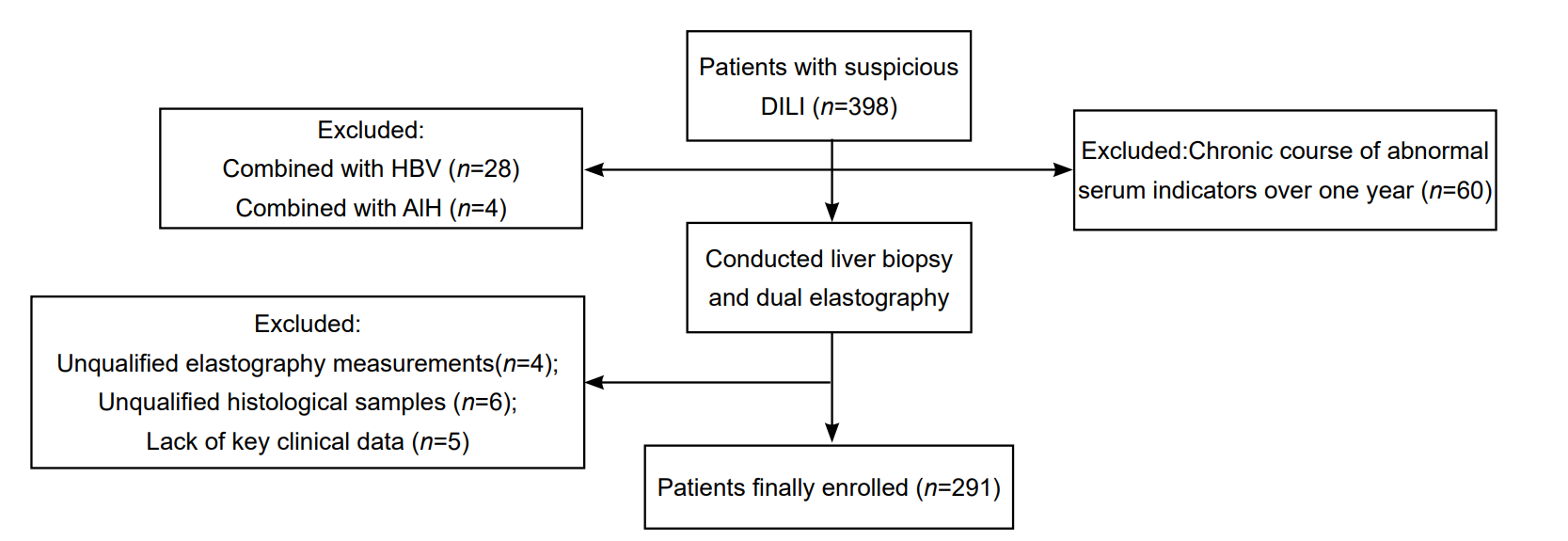
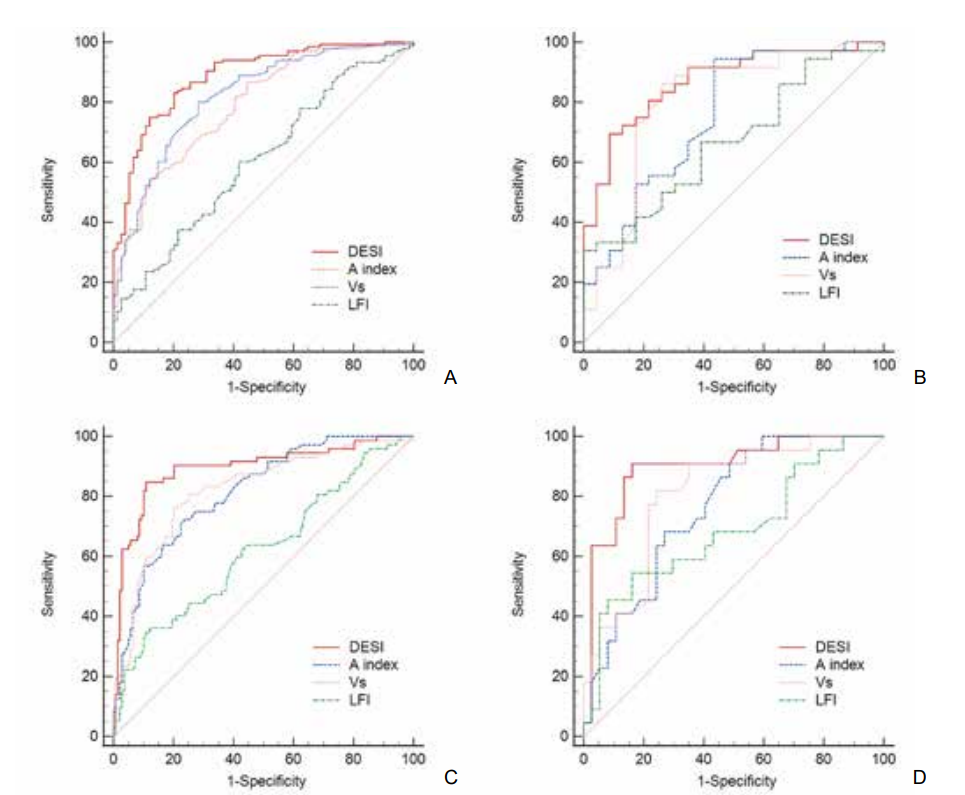
| [1] |
Vuppalanchi R, Ghabril M. Review article: clinical assessment of suspected drug-induced liver injury and its management. Aliment Pharmacol Ther 2022;56:1516-1531.
|
| [2] |
Kobayashi T, Iwaki M, Nogami A, Yoneda M. Epidemiology and management of drug-induced liver injury: importance of the updated RUCAM. J Clin Transl Hepatol 2023;11:1239-1245.
|
| [3] |
Allison R, Guraka A, Shawa IT, Tripathi G, Moritz W, Kermanizadeh A. Drug induced liver injury - a 2023 update. J Toxicol Environ Health B Crit Rev 2023;26:442-467.
|
| [4] |
Ma ZT, Shi Z, Xiao XH, Wang JB. New insights into herb-induced liver injury. Antioxid Redox Signal 2023;38:1138-1149.
|
| [5] |
Bjornsson H K, Bjornsson E S. Drug-induced liver injury: Pathogenesis, epidemiology, clinical features, and practical management. Eur J Intern Med 2022;97:26-31.
|
| [6] |
Garcia-Cortes M, Robles-Diaz M, Stephens C, Ortega-Alonso A, Lucena MI, Andrade RJ. Drug induced liver injury: an update. Arch Toxicol 2020;94:3381-3407.
|
| [7] |
Clinton JW, Kiparizoska S, Aggarwal S, Woo S, Davis W, Lewis JH. Drug-induced liver injury: highlights and controversies in the recent literature. Drug Saf 2021;44:1125-1149.
|
| [8] |
Fontana RJ, Liou I, Reuben A, Suzuki A, Fiel MI, Lee W, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology 2023;77:1036-1065.
|
| [9] |
Ginès P, Castera L, Lammert F, Graupera I, Serra-Burriel M, Allen AM, et al. Population screening for liver fibrosis: Toward early diagnosis and intervention for chronic liver diseases. Hepatology 2022;75:219-228.
|
| [10] |
Kennedy P, Wagner M, Castéra L, Hong CW, Johnson CL, Sirlin CB, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology 2018;286:738-763.
|
| [11] |
Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med 2017;38:e16-e47.
|
| [12] |
Yada N, Sakurai T, Minami T, Arizumi T, Takita M, Hagiwara S, et al. Influence of liver inflammation on liver stiffness measurement in patients with autoimmune hepatitis evaluation by combinational elastography. Oncology 2017;92Suppl 1:10-15.
|
| [13] |
Zhao Y, Wu L, Qin H, Li Q, Shen C, He Y, et al. Preoperative combi-elastography for the prediction of early recurrence after curative resection of hepatocellular carcinoma. Clin Imaging 2021;79:173-178.
|
| [14] |
Medina-Caliz I, Robles-Diaz M, Garcia-Muñoz B, Stephens C, Ortega-Alonso A, Garcia-Cortes M, et al. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol 2016;65:532-542.
|
| [15] |
Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am J Gastroenterol 2021;116:878-898.
|
| [16] |
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;89:806-815.
|
| [17] |
Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the society of radiologists in ultrasound liver elastography consensus statement. Radiology 2020;296:263-274.
|
| [18] |
Yada N, Kudo M, Morikawa H, Fujimoto K, Kato M, Kawada N. Assessment of liver fibrosis with real-time tissue elastography in chronic viral hepatitis. Oncology 2013;84Suppl 1:13-20.
|
| [19] |
Yada N, Tamaki N, Koizumi Y, Hirooka M, Nakashima O, Hiasa Y, et al. Diagnosis of fibrosis and activity by a combined use of strain and shear wave Imaging in patients with liver disease. Dig Dis 2017;35:515-520.
|
| [20] |
Caballero T, Pérez-Milena A, Masseroli M, O'Valle F, Salmerón FJ, Del Moral RM, et al. Liver fibrosis assessment with semiquantitative indexes and image analysis quantification in sustained-responder and non-responder interferon-treated patients with chronic hepatitis C. J Hepatol 2001;34:740-747.
|
| [21] |
Jianping D, Xi C, Guangwen C, Fankun M, Ying Z, Bulin Z, et al. Dual elastography to discriminate adjacent stages of fibrosis and inflammation in chronic hepatitis B: A prospective multicenter study. Hepatology 2024;79:438-450.
|
| [22] |
Mcgill MR, Jaeschke H. Biomarkers of drug-induced liver injury: progress and utility in research, medicine, and regulation. Expert Rev Mol Diagn 2018;18:797-807.
|
| [23] |
Rathi C, Pipaliya N, Patel R, Ingle M, Phadke A, Sawant P. Drug induced liver injury at a tertiary hospital in India: etiology, clinical features and predictors of mortality. Ann Hepatol 2017;16:442-450.
|
| [24] |
Ahmad J, Barnhart HX, Bonacini M, Ghabril M, Hayashi PH, Odin JA, et al. Value of liver biopsy in the diagnosis of drug-induced liver injury. J Hepatol 2022;76:1070-1078.
|
| [25] |
Li ZB, Chen DD, He QJ, Li L, Zhou G, Fu YM, et al. The LAC score indicates significant fibrosis in patients with chronic drug-induced liver injury: A large biopsy-based study. Front Pharmacol 2021;12:734090.
|
| [26] |
Rongngern P, Chularojanamontri L, Wongpraparut C, Silpa-Archa N, Chotiyaputta W, Pongpaibul A, et al. Diagnostic performance of transient elastography for detection of methotrexate-induced liver injury using Roenigk classification in Asian patients with psoriasis: a retrospective study. Arch Dermatol Res 2017;309:403-408.
|
| [27] |
Fu H, Shen Z, Lai R, Zhou T, Huang Y, Zhao S, et al. Clinic-radiomics model using liver magnetic resonance imaging helps predict chronicity of drug-induced liver injury. Hepatol Int 2023;17:1626-1636.
|
| [28] |
Wu T, Yang D, Wee A, Wang Y, Li M, Liu J, et al. Identification of MRI features associated with injury type, severity, and prognosis in drug-induced liver injury. Eur Radiol 2023;33:666-677.
|