Advanced Ultrasound in Diagnosis and Therapy ›› 2025, Vol. 9 ›› Issue (4): 357-374.doi: 10.26599/AUDT.2025.250102
Previous Articles Next Articles
Xu Junmeia, Tahmasebi Aylina, Mohammed Amra, Pour Bahareh Kiana, Liu Ji-Bina, Eisenbrey John R.a,*( )
)
Received:2025-09-25
Revised:2025-10-13
Accepted:2025-10-21
Online:2025-12-30
Published:2025-11-06
Contact:
Department of Radiology, Thomas Jefferson University, 132 South 10th St, Philadelphia PA, USA (John R. Eisenbrey),e-mail: John.Eisenbrey@jefferson.edu (JR E).,
Xu Junmei, Tahmasebi Aylin, Mohammed Amr, Pour Bahareh Kian, Liu Ji-Bin, Eisenbrey John R.. Contrast-enhanced Ultrasound LI-RADS for Nonradiation Treatment Response Assessment in Liver Tumor: A Pictorial Review Based on LR-TR v2024. Advanced Ultrasound in Diagnosis and Therapy, 2025, 9(4): 357-374.

Figure 2
ACR CEUS LR-TR categories v2024 [27]. This flowchart illustrates the stepwise assessment of both intralesional and perilesional tumor viability using CEUS imaging criteria. Color-coded boxes indicate the final LR-TR categories, including green=Nonviable, yellow=Equivocal, and red=Viable. The algorithm incorporates a tiebreaking rule and reconciliation process to assign a single TRA category. Applicable for treated lesions after transarterial embolization (cTACE/DEB-TACE/TAE) and percutaneous ablation (RFA/MWA/PEI)."
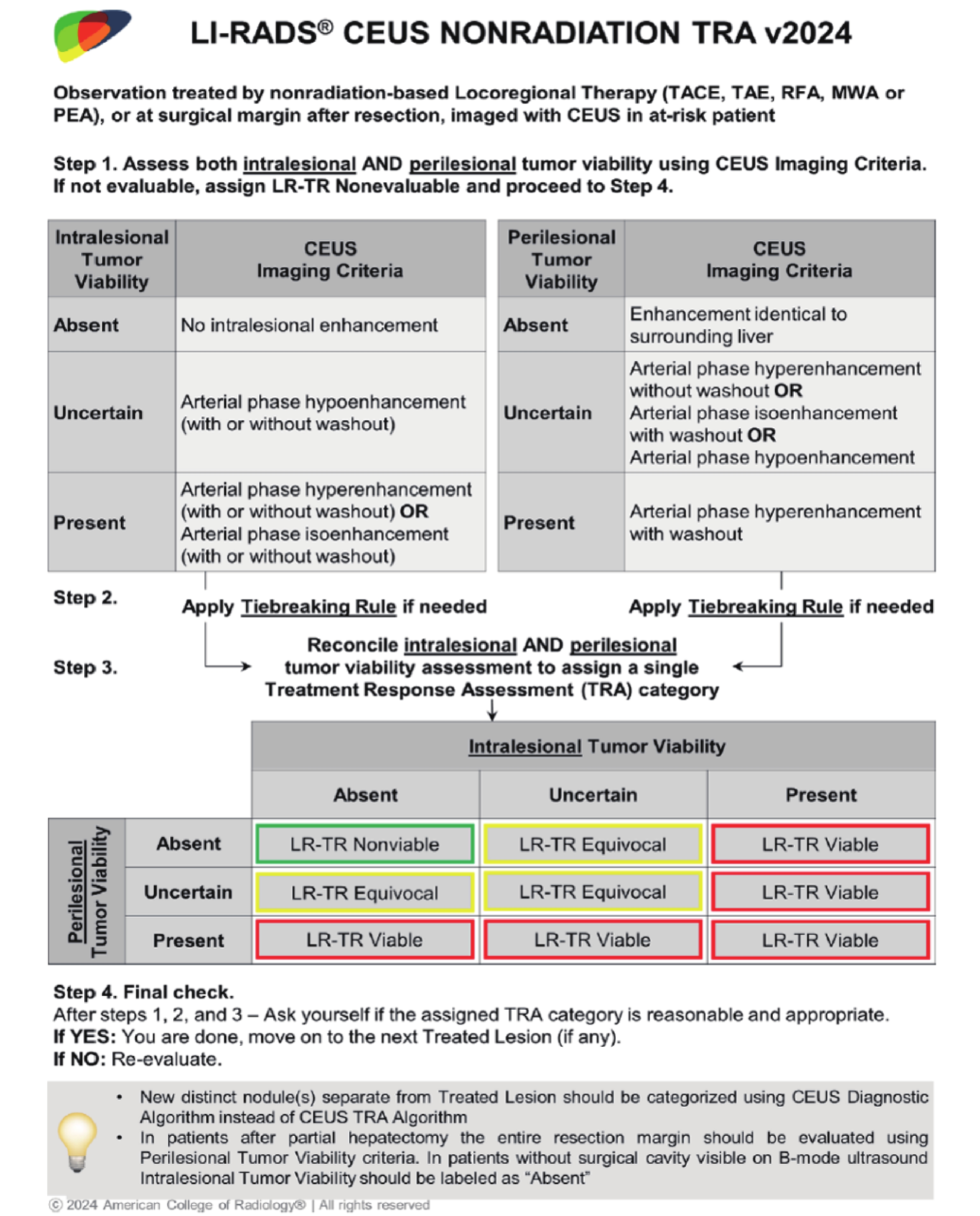

Figure 3
Flowchart in NLT with CEUS assessment covered by LR-TR v2024. CEUS, contrast-enhanced ultrasound; LR-TR, liver imaging reporting and data system treatment response algorithm; NLT, nonradiation locoregional therapy; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; TAE, bland transarterial embolization; RFA, radiofrequency ablation; MWA, microwave ablation; PEI, percutaneous ethanol injection; HCC, hepatocellular carcinoma; BCLC-B, Barcelona Clinic Liver Cancer stage B."
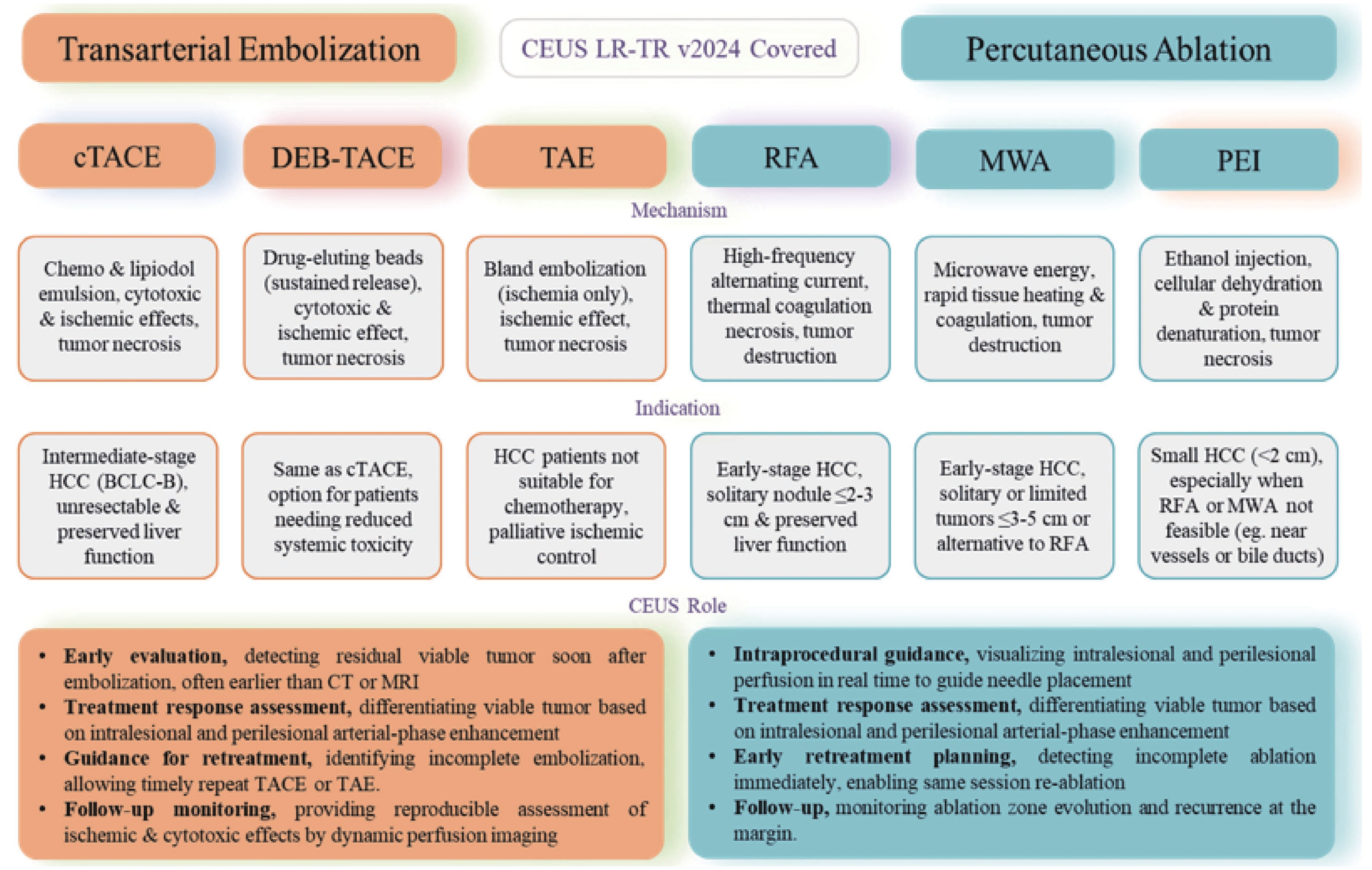
Figure 4
LR-TR Nonviable. Example of a nonviable tumor 14 days after TACE. (A) B-mode image shows two heterogeneous hyperechoic lesions (thick arrow) with irregular shape, measuring 17 mm and 18 mm in segment VI; (B-E) CEUS shows no intralesional enhancement with perilesional enhancement identical to the surrounding liver parenchyma in all phases. Findings are consistent with LR-TR Nonviable."
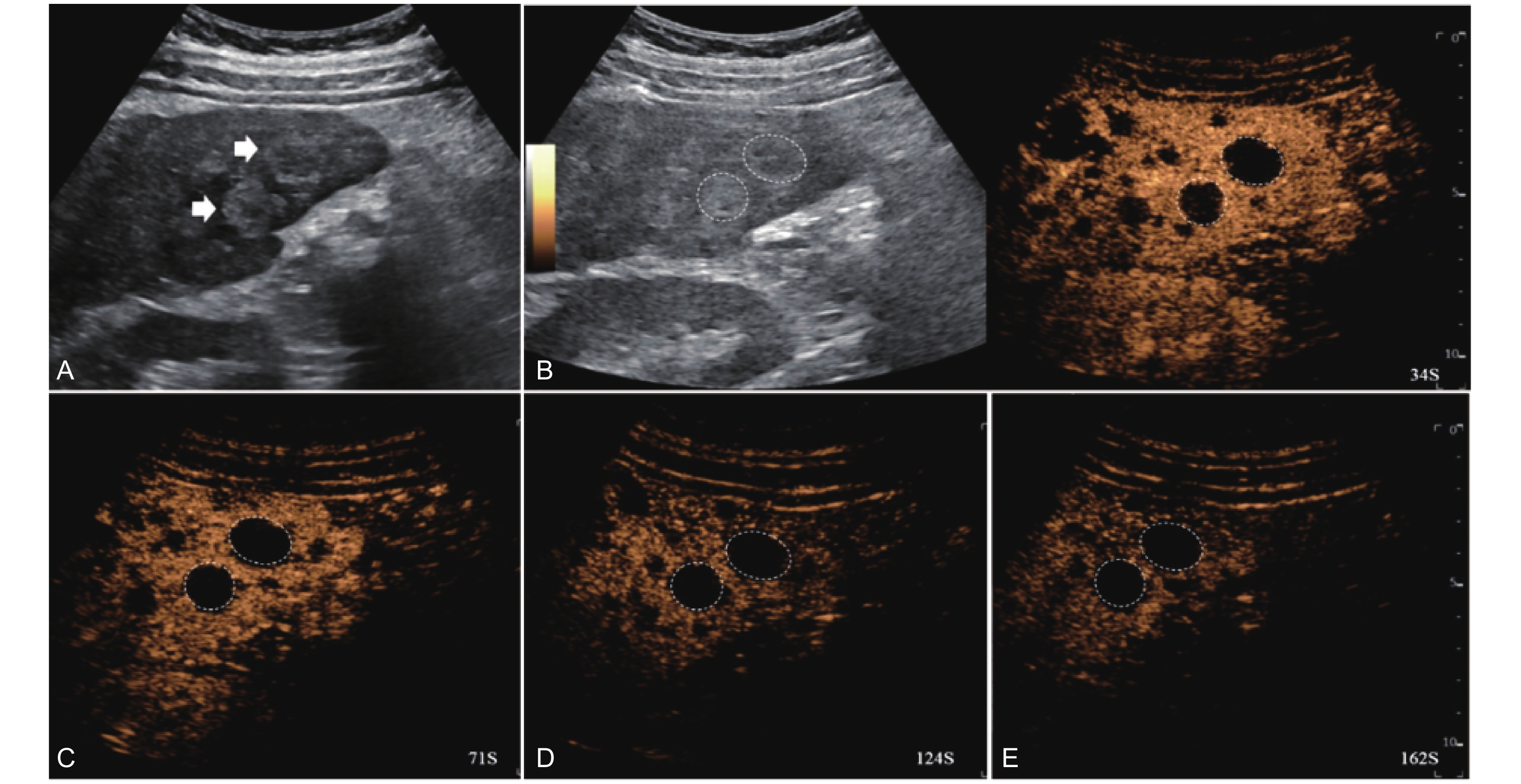
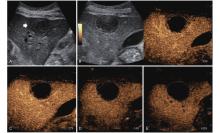
Figure 5
LR-TR Equivocal. Example of a treated tumor 36 days after TACE. (A) B-mode image shows a hypoechoic lesion (thick arrow) with ill-defined margins, measuring 36 mm in segment V; (B-C) CEUS shows intralesional ringlike hypoenhancement with early mild washout in the arterial phase; (D) The lesion shows ringlike hypoenhancement with marked washout in the portal venous phase; (E) The lesion shows complete washout in the late phase. Perilesional enhancement remains identical to the surrounding liver parenchyma throughout all phases. Findings are consistent with LR-TR Equivocal."
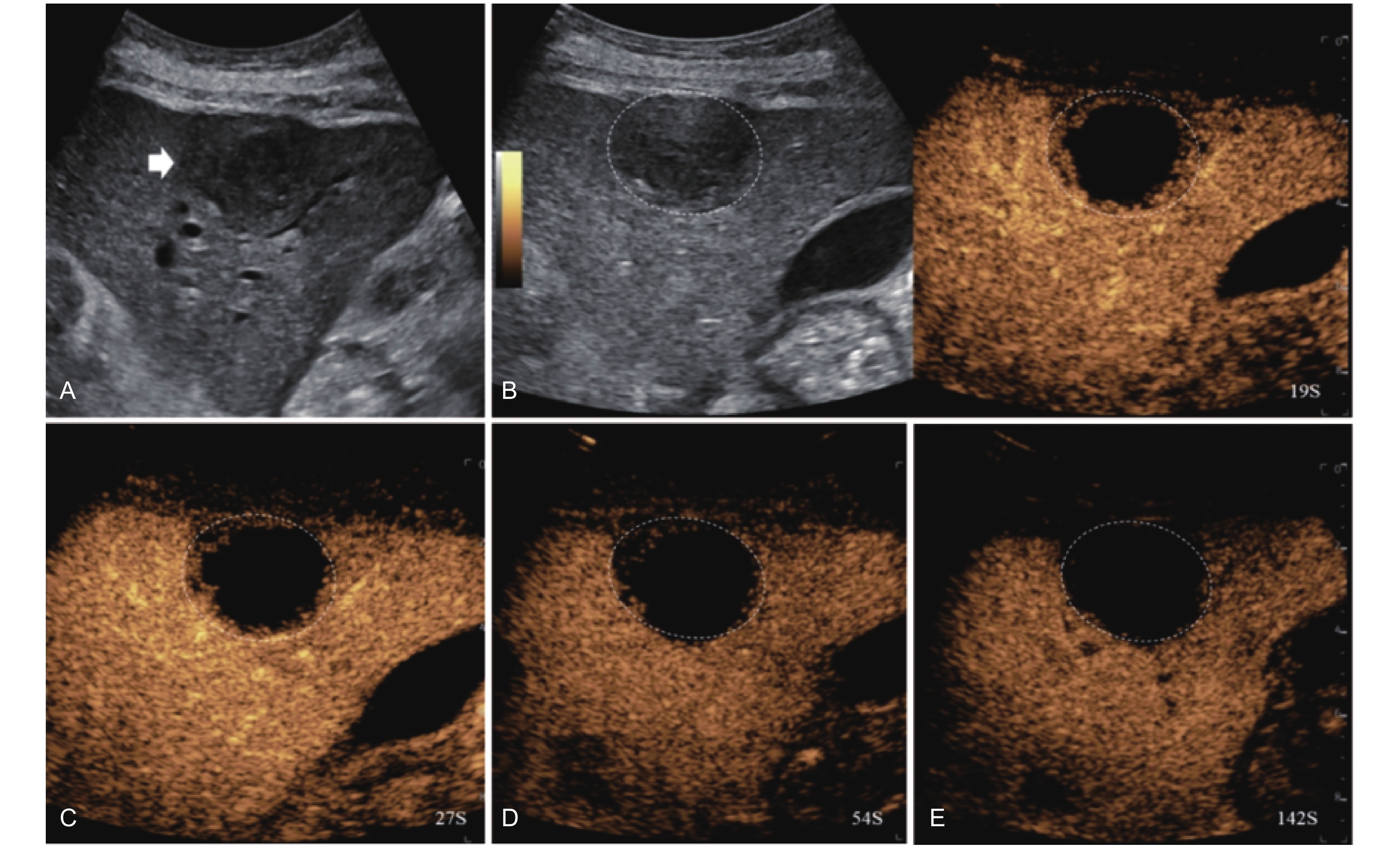
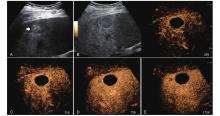
Figure 6
LR-TR Equivocal. Example of a treated tumor 16 days after TACE. (A) B-mode image a heterogeneous hyperechoic lesion (thick arrow) with well-defined margins, measuring 32 mm in segment VIII; (B-C) CEUS shows no intralesional enhancement with perilesional hyperenhancement in the arterial phase; (D-E) The lesion remains no intralesional enhancement with perilesional identical to the surrounding liver parenchyma without washout in the portal venous and late phases. Findings are consistent with LR-TR Equivocal."
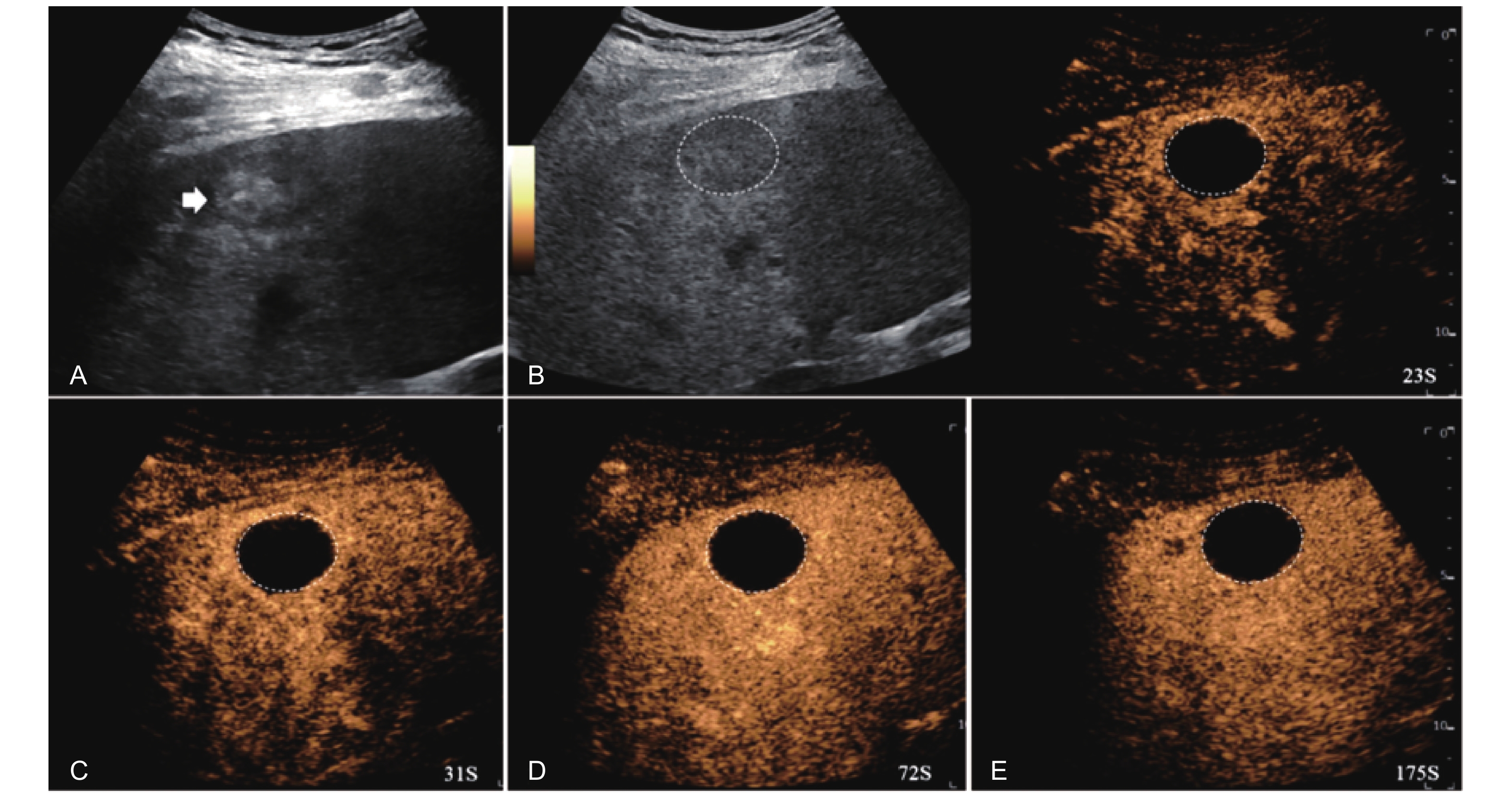
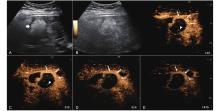
Figure 7
LR-TR Equivocal. Example of a treated tumor 22 days after TACE. (A) B-mode image shows a hyperechoic lesion (thick arrow) with well-defined margins, measuring 47 mm in segment VIII; (B-C) CEUS shows intralesional hypoenhancement with nodular protrusions along the margin and early marked washout during the arterial phase; (D-E) The lesion shows complete washout in the portal venous and late phases. Perilesional APHE persists without washout throughout all phases (thin arrow). Findings are consistent with LR-TR Equivocal."
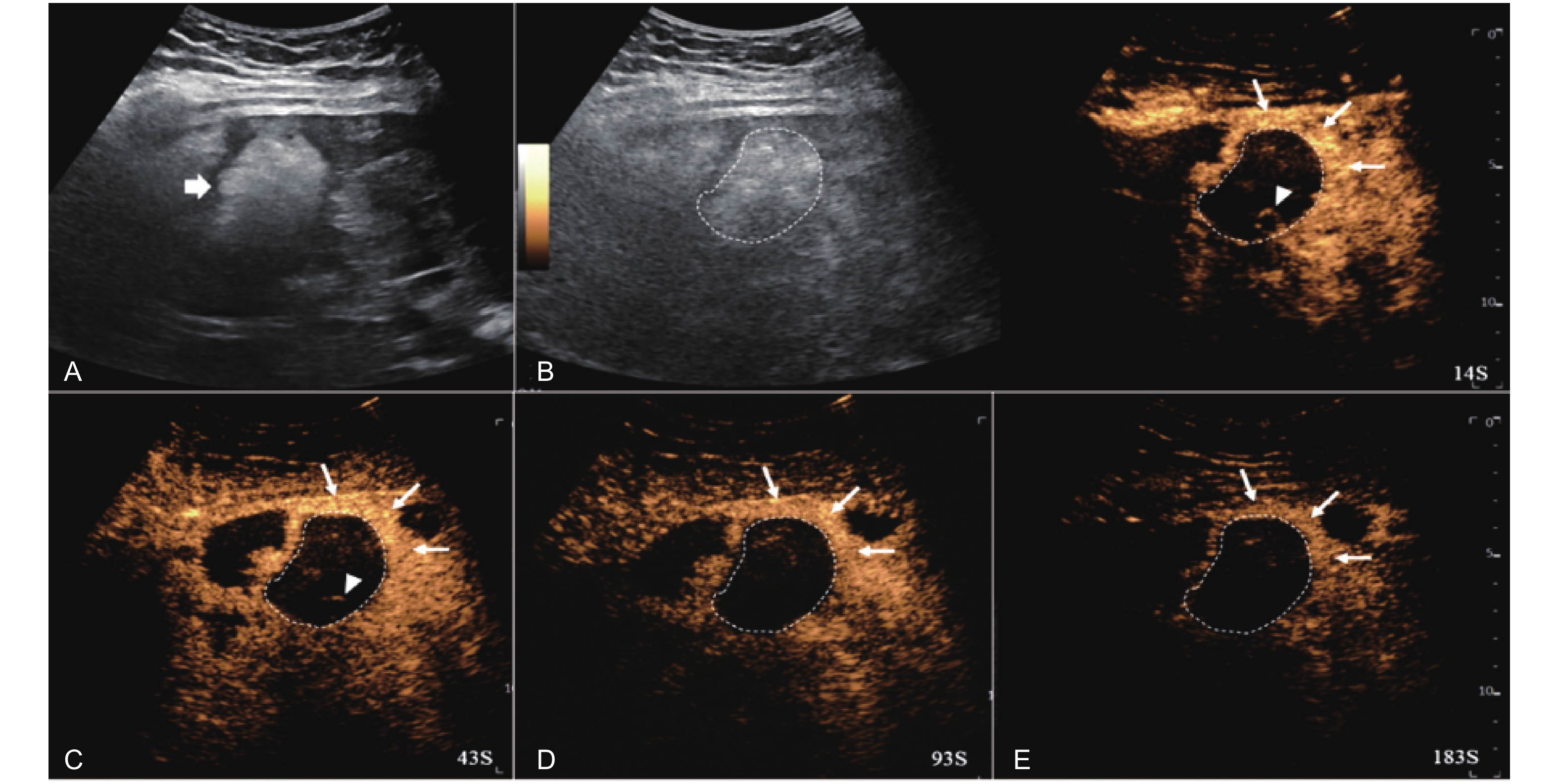
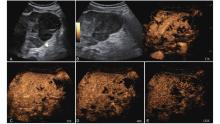
Figure 8
LR-TR Viable. Example of a treated tumor 36 days after TACE. (A) B-mode image shows a heterogeneous echogenic lesion (thick arrow) with irregular shape, measuring 115 mm in segment II and III; (B-C) CEUS shows intralesional heterogeneous hyperenhancement during the arterial phase; (D-E) The lesion shows partial mild washout in the portal venous and late phases. Perilesional enhancement remains identical to the surrounding liver parenchyma throughout all phases. Findings are consistent with LR-TR Viable."
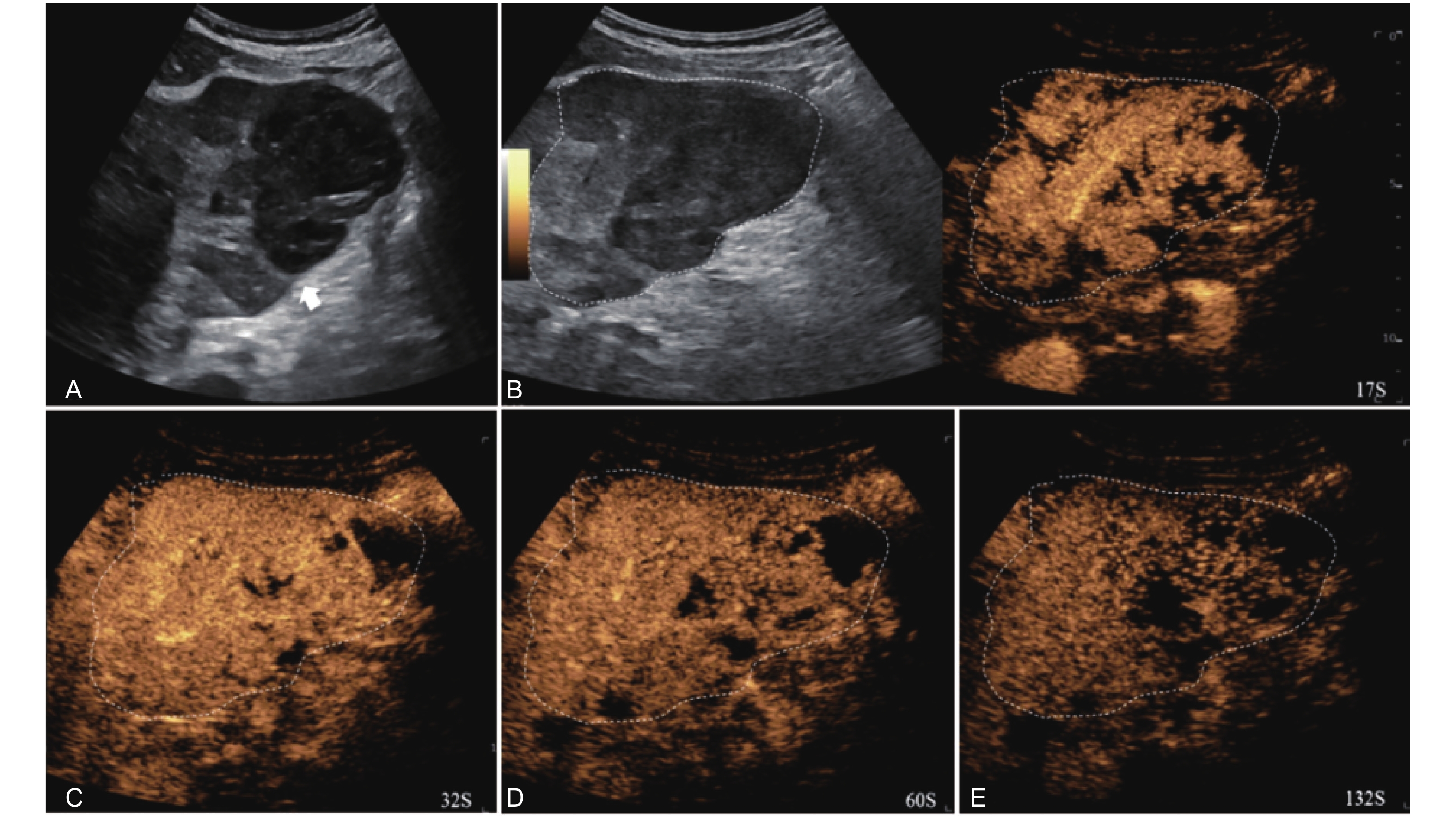
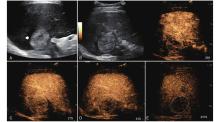
Figure 9
LR-TR Viable. Example of a treated tumor 13 days after TACE. (A) B-mode image shows a hyperechoic lesion (thick arrow) with well-defined margins, measuring 46 mm in segment VI; (B-C) CEUS shows intralesional heterogeneous hyperenhancement and early mild washout during the arterial phase; (D-E) The lesion shows marked washout in the portal venous and late phases. Perilesional enhancement remains identical to the surrounding liver parenchyma throughout all phases. Findings are consistent with LR-TR Viable."
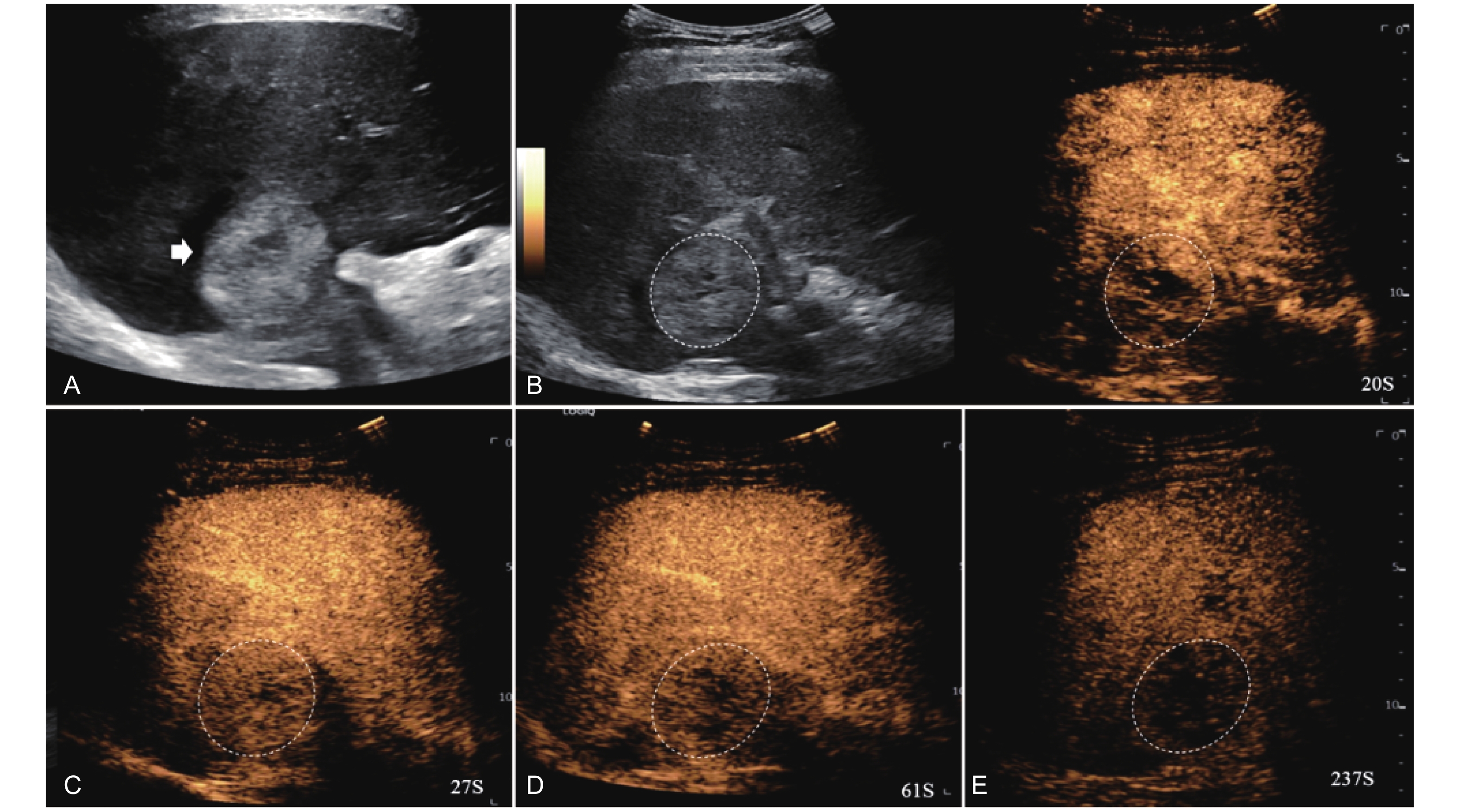
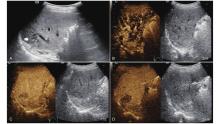
Figure 10
LR-TR Viable. A 56-year-old male with HCC, 1 year after RF ablation. (A) B-mode image shows a heterogeneous mildly hypoechoic lesion (thick arrow) with poor-defined margins in segment VII; (B) Post-treatment CEUS shows intralesional heterogeneous hyperenhancement; (C) The lesion shows mild washout in the portal venous phase; (D) The lesion shows marked washout in the late phase. Perilesional enhancement remains identical to the surrounding liver parenchyma throughout all phases. Findings are consistent with LR-TR Viable. (This case and figures were contributed by Dr. Qiang Lu from Huaxi Hospital of Sichuan University)"
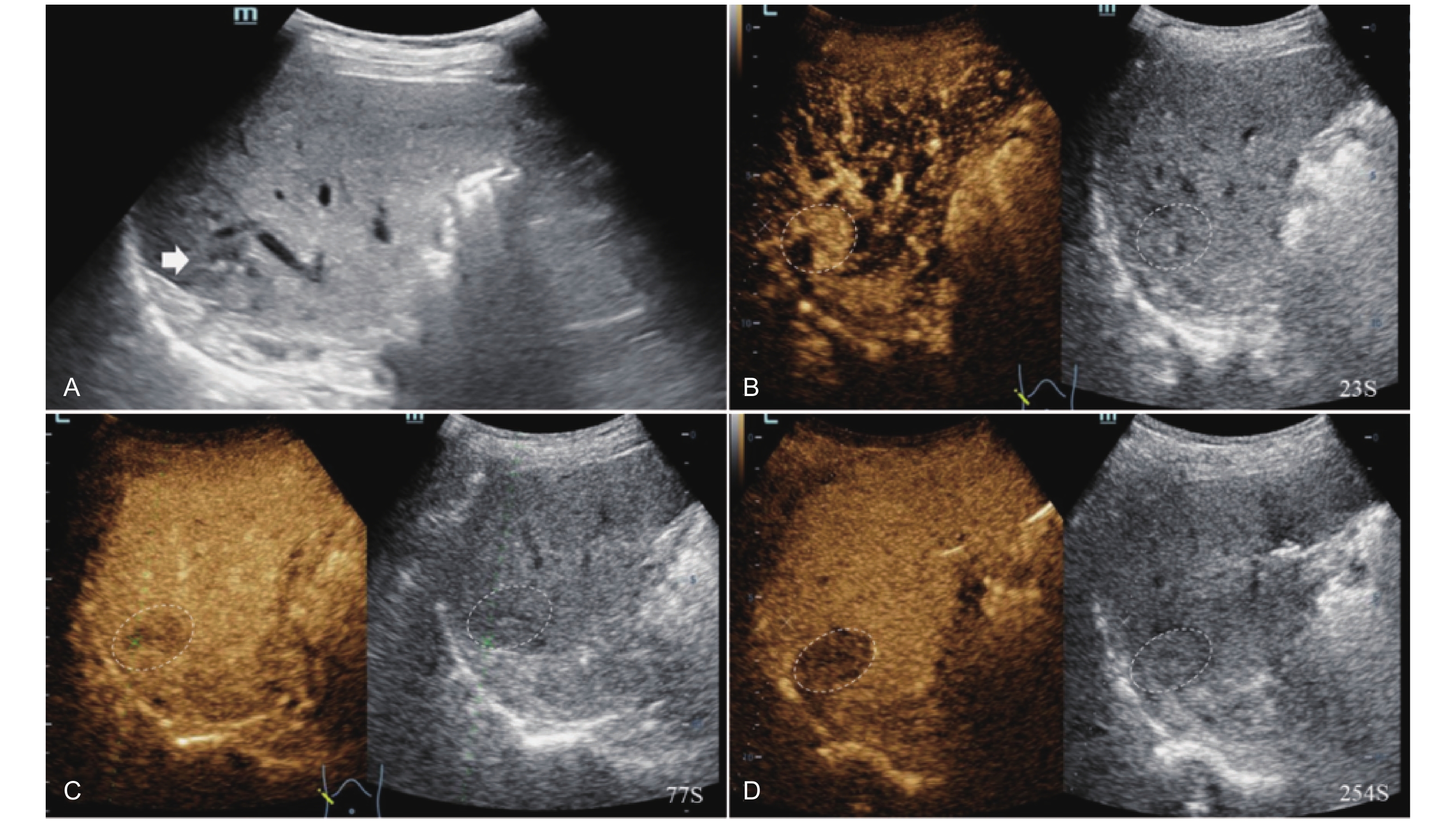
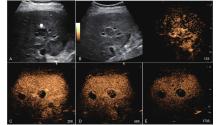
Figure 11
LR-TR Viable. Example of a treated tumor 49 days after TACE. (A) B-mode image shows a hypoechoic lesion (thick arrow) with well-defined margins, measuring 22 mm in segment IV; (B-C) CEUS shows intralesional heterogeneous hyperenhancement with early marked washout and perilesional enhancement identical to the surrounding liver parenchyma during the arterial phase; (D) The lesion shows marked washout with perilesional mild hyperenhancement in the portal venous phase; (E) The lesion shows marked washout with partial perilesional mild washout in late phase. Findings are consistent with LR-TR Viable."
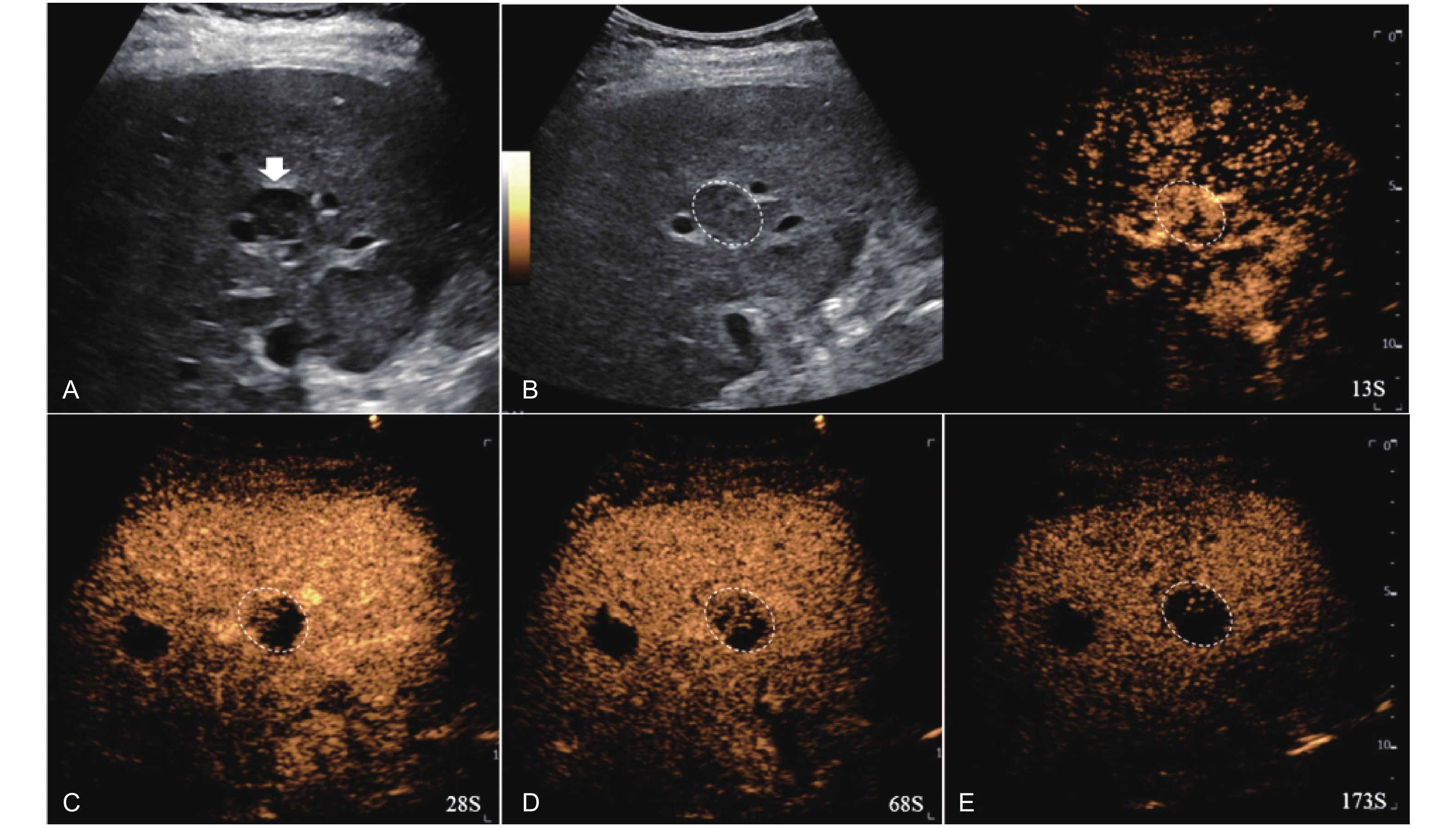
Figure 12
LR-TR Viable. Example of a treated tumor 55 days after TACE. (A) B-mode image shows a hypoechoic lesion (thick arrow) with well-defined margins, measuring 52 mm in segment III; (B) CEUS shows intralesional hyperenhancement with nodular protrusions along the margin (arrowhead) and perilesional hyperenhancement (thin arrow) during the arterial phase; (C-D) The lesion shows intralesional (arrowhead) and perilesional isoenhancement (thin arrow) without washout in the portal venous and late phases. Findings are consistent with LR-TR Viable."
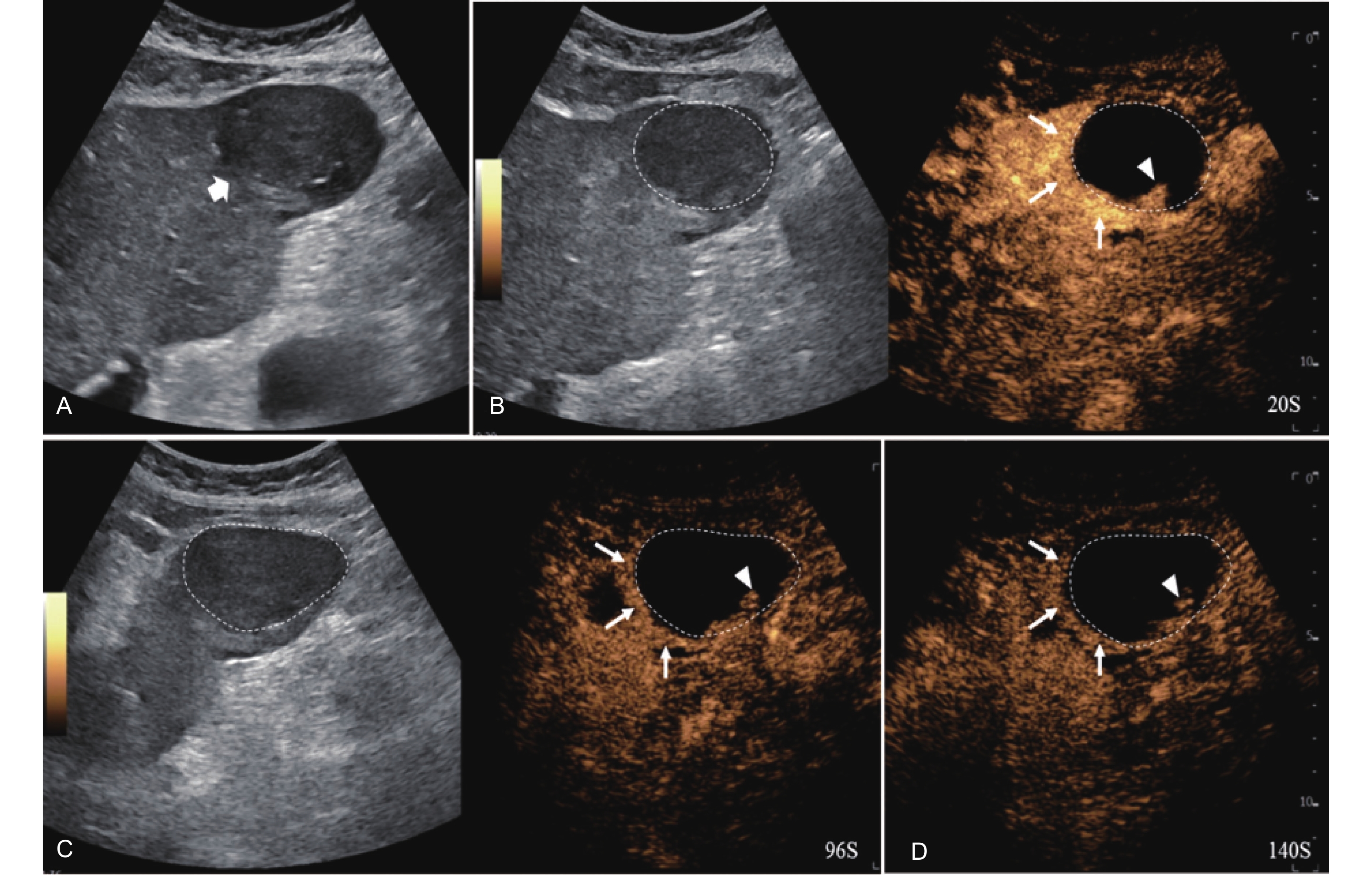
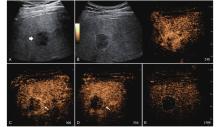
Figure 13
LR-TR Viable. Example of a treated tumor 46 days after TACE. (A) B-mode image shows a hypoechoic lesion (thick arrow) with ill-defined margins, measuring 22 mm in segment VIII; (B-C) CEUS shows intralesional hyperenhancement with early marked washout and perilesional hyperenhancement during the arterial phase; (D-E) The lesion shows intralesional marked washout and perilesional partial mild washout (thin arrow) in the portal venous and late phases. Findings are consistent with LR-TR Viable."
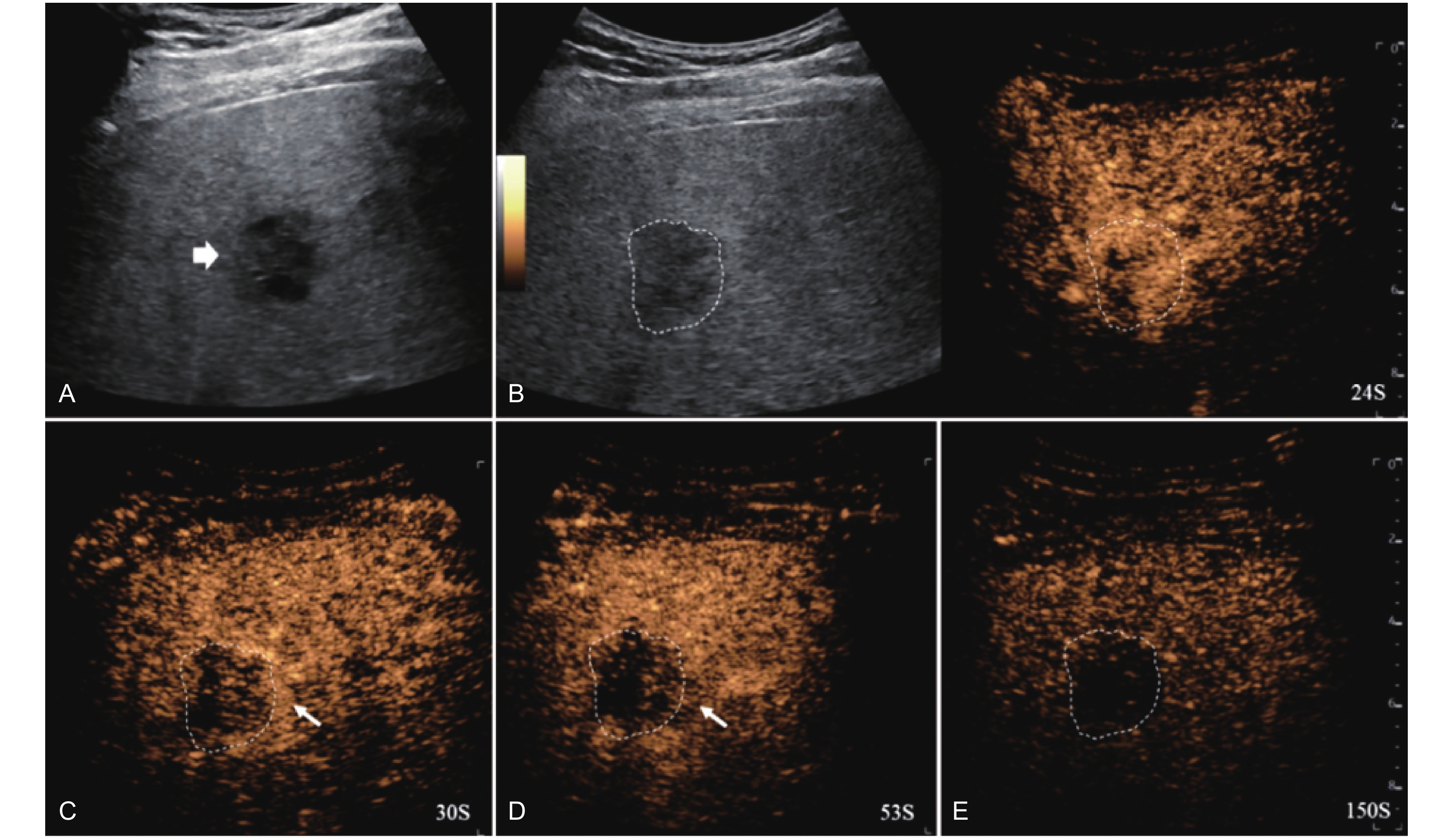
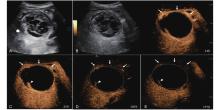
Figure 14
LR-TR Viable. Example of a treated tumor 16 days after TACE. (A) B-mode image shows a cystic-solid heterogeneous echogenic lesion with a peripheral hypoechoic band (thick arrow), measuring 71 mm in segment V and VI; (B-C) CEUS shows intralesional partial hyperenhancement (arrowhead) with perilesional hyperenhancement (thin arrow) during the arterial phase; (D-E) The lesion shows intralesional marked washout (arrowhead) and perilesional partial marked washout (thin arrow) in the portal venous and late phases. Findings are consistent with LR-TR Viable."
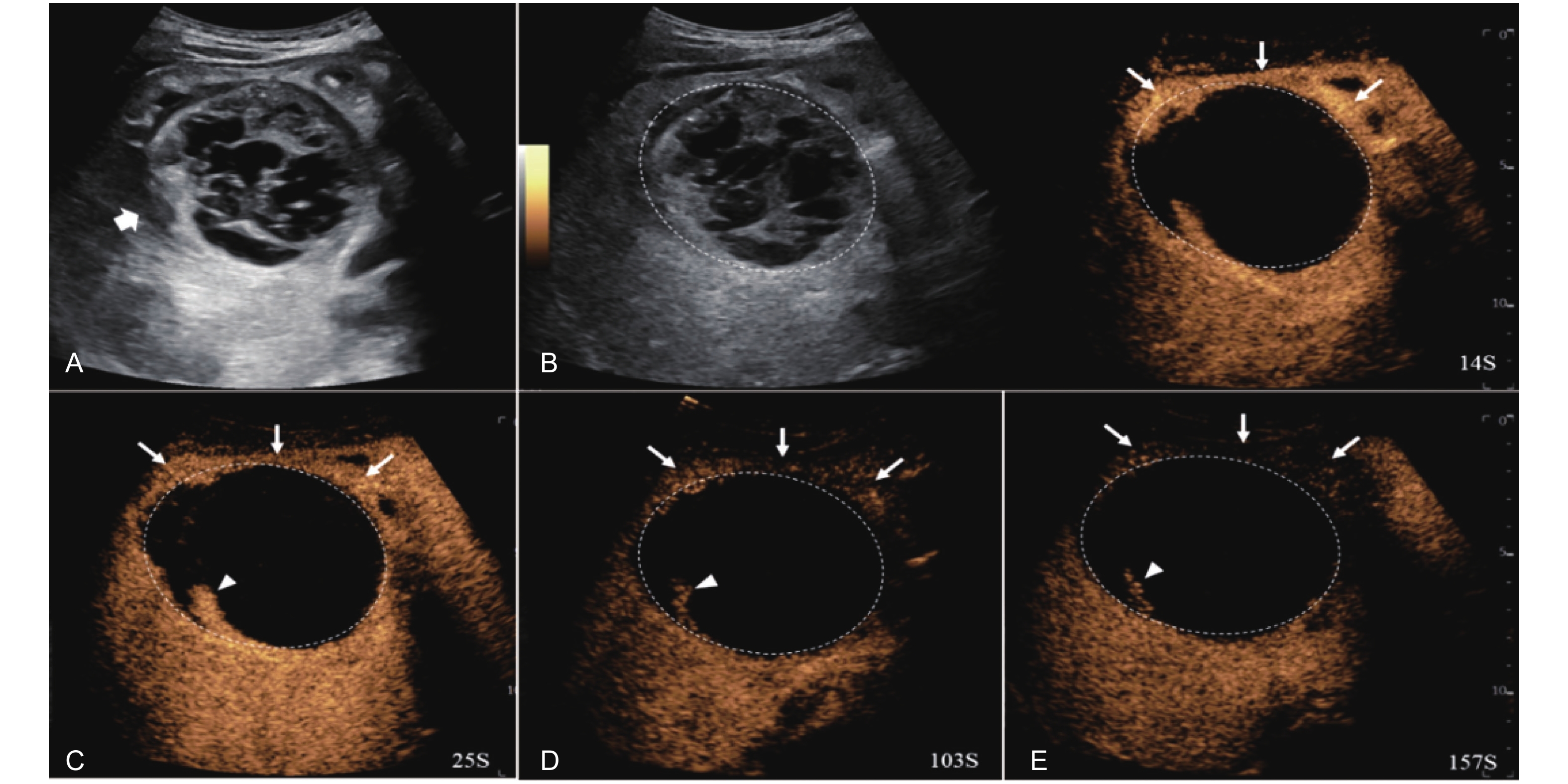
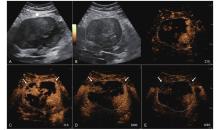
Figure 15
LR-TR Viable. Example of a treated tumor 29 days after TACE. (A) B-mode image shows a heterogeneous mildly hypoechogenic lesion with well-defined margins (thick arrow), measuring 85 mm in segment II and III; (B-C) CEUS shows partial intralesional hyperenhancement with perilesional hyperenhancement (thin arrow) during the arterial phase; (D-E) The lesion shows intralesional and perilesional (thin arrow) gradual washout in the portal venous and late phases. Findings are consistent with LR-TR Viable."
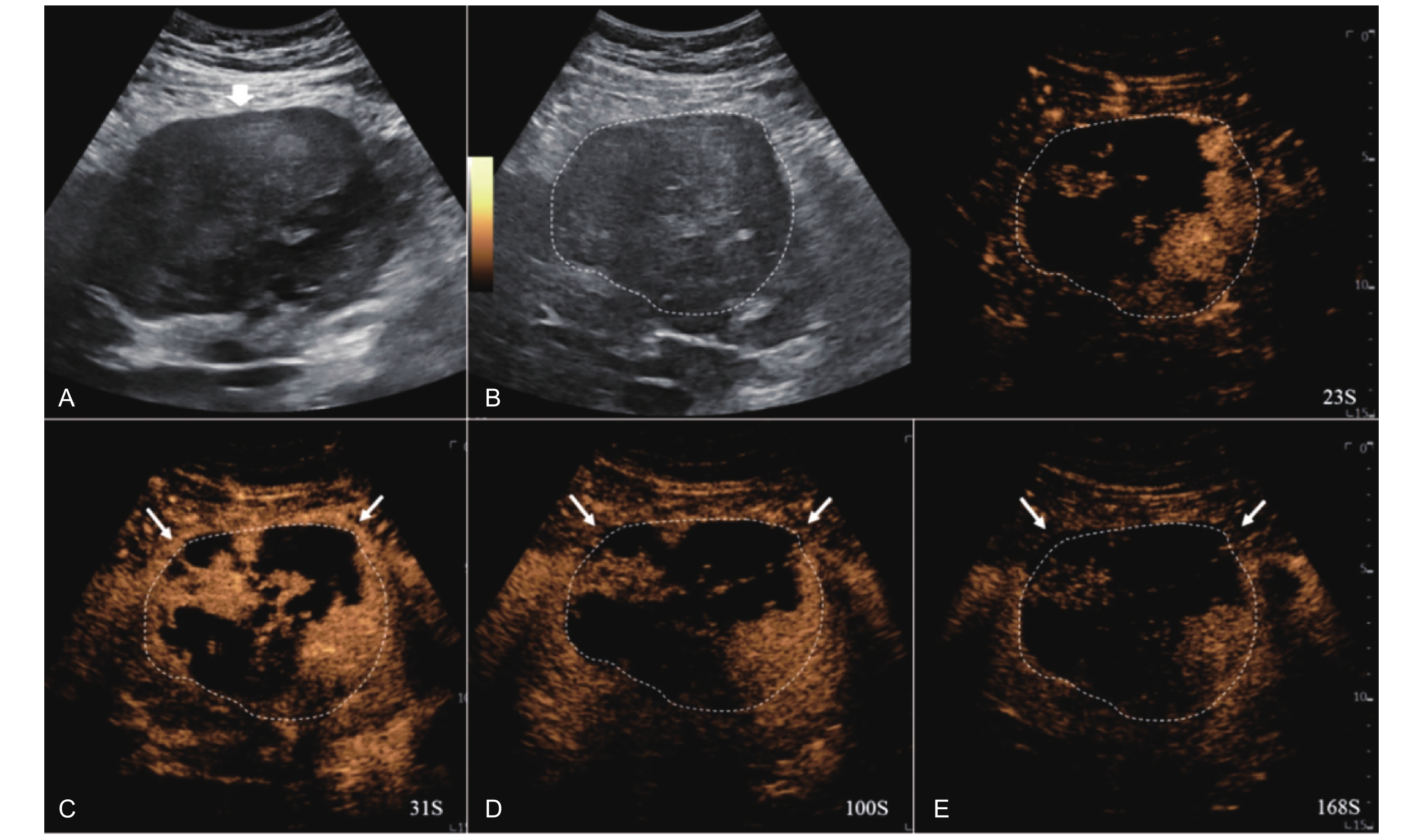
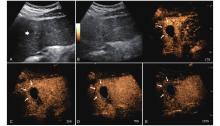
Figure 16
LR-TR Viable. Example of a treated tumor 15 days after TACE. (A) B-mode image shows slightly hyperechoic lesion with well-defined margins (thick arrow), measuring 22 mm in segment IV; (B-D) CEUS shows perilesional hyperenhancement (thin arrow) during the arterial and portal venous phases; (E) The lesion shows perilesional marked washout (thin arrow) in the late phase. No intralesional enhancement throughout all phases. Findings are consistent with LR-TR Viable."
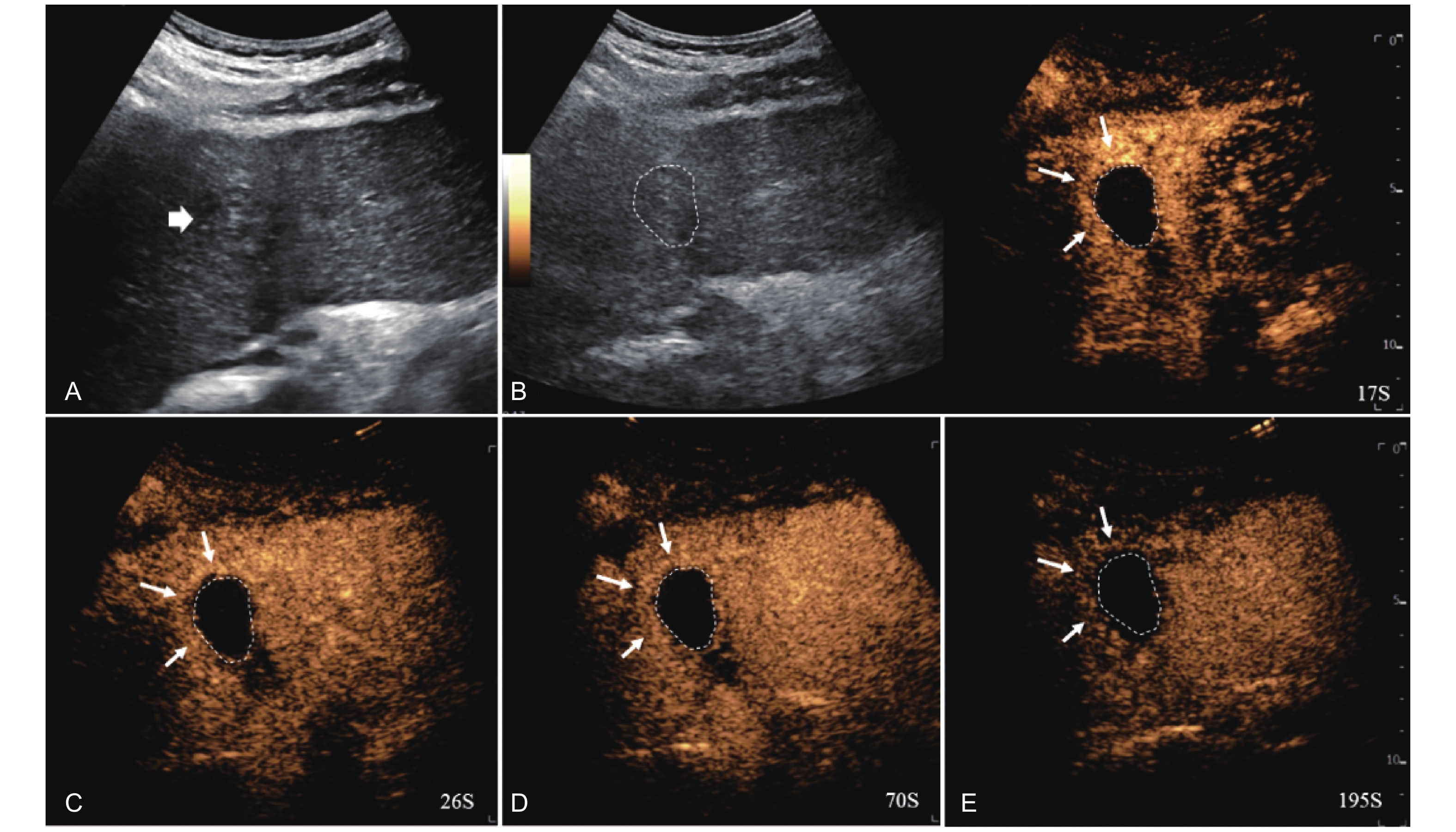
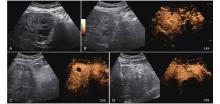
Figure 17
LR-TR Nonevaluable. Example of a treated tumor 22 days after TACE. (A) B-mode image shows a slightly hyperechoic target lesion measuring 24 mm in segment IV; (B) CEUS shows mild hypoenhancement of the lesion at 14 s during the arterial phase; (C) A non enhancing lesion measuring 16 mm on US distinct from the target lesion is observed at 24 s of the arterial phase; (D) The lesion is not visualized in the portal venous phase. This finding represents inadequate arterial-phase coverage and is defined as a nonevaluable lesion."
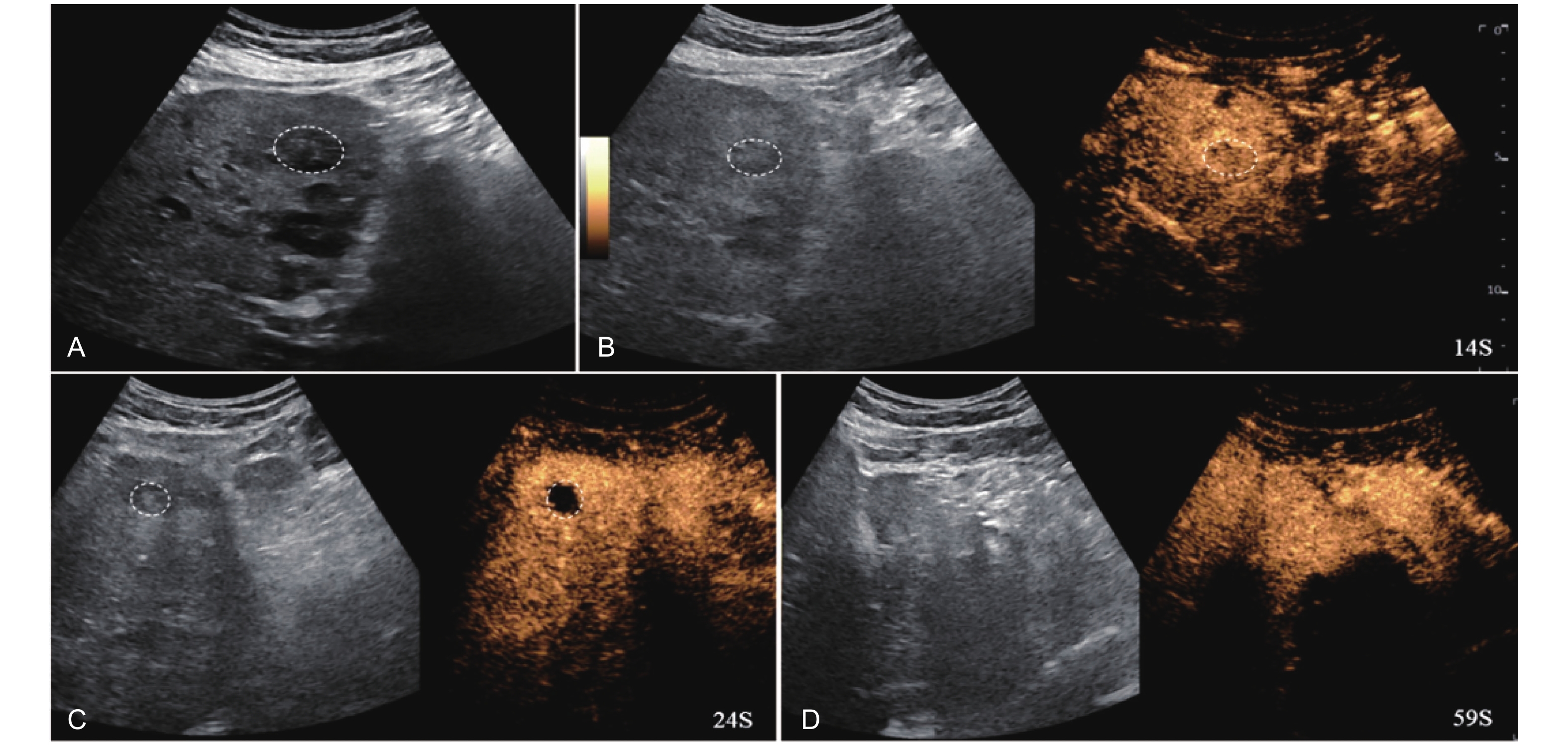
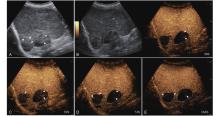
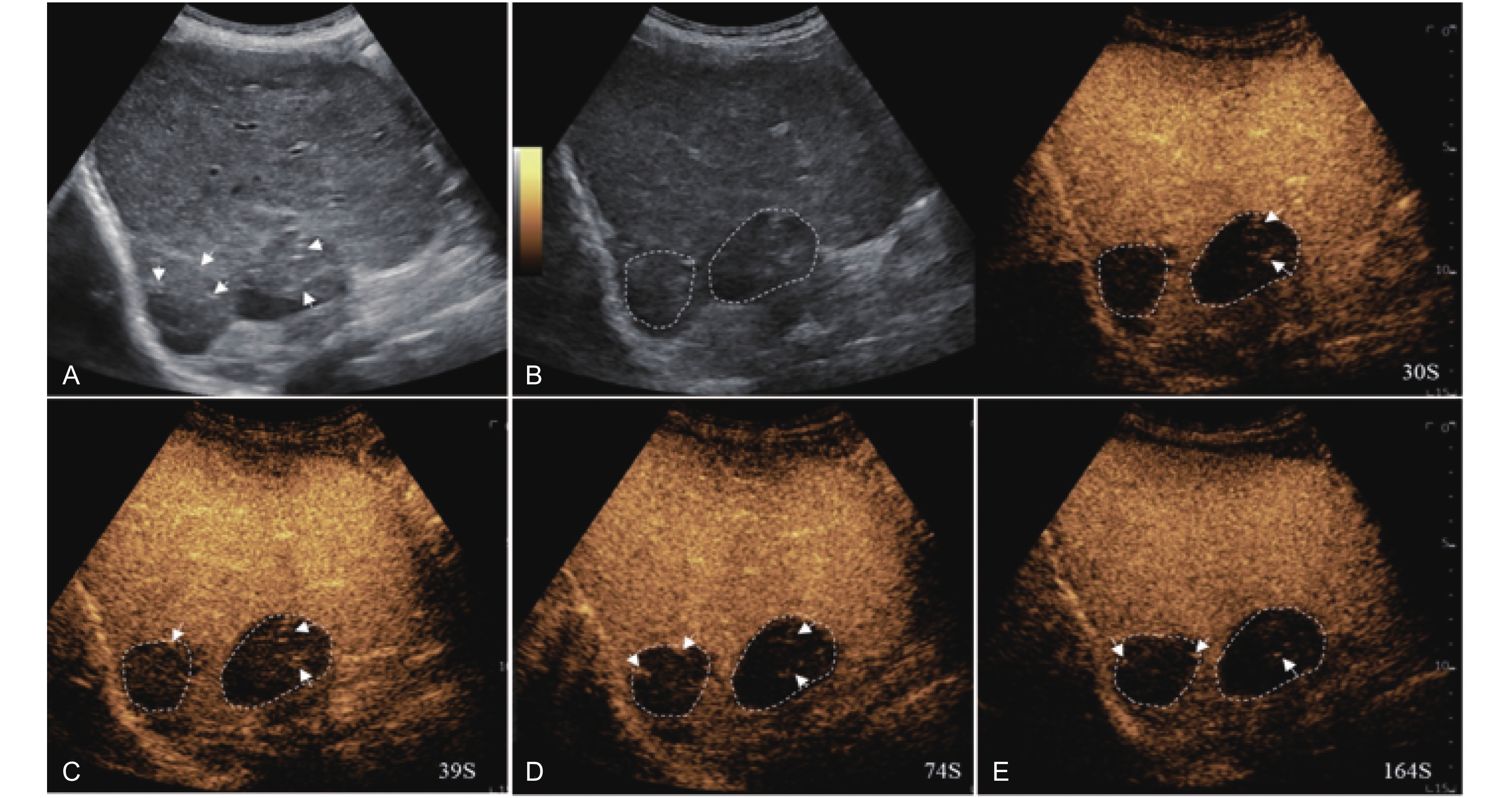
Figure 18
Artifact. Example of a treated tumor 101 days after TACE. (A) B-mode image shows two slightly hyperechoic lesions with scattered punctate calcifications (short arrow), measuring approximately 38 mm and 32 mm in segment VI and VII; (B-E) CEUS shows persistent intralesional flocculent hypoenhancement with punctate hyper- or isoenhancement (short arrow) throughout all phases, without evidence of contrast diffusion. This finding is considered an artifact."
| [1] | Sung H , Ferlay J , Siegel RL , Laversanne M , Soerjomataram I , Jemal A , et al . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209-249 |
| [2] | Torre LA , Bray F , Siegel RL , Ferlay J , Lortet-Tieulent J , Jemal A . Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87-108 |
| [3] | Josep ML , Jessica ZR , Eli P , Bruno S , Myron S , Morris S , et al . Hepatocellular carcinoma. Nat Rev Dis Primers 2016; 14: 16018 |
| [4] | Westwood M , Joore M , Grutters J , Redekop K , Armstrong N , Lee K , et al . Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess 2013; 17: 1-243 |
| [5] |
Tanaka H , Iijima H , Nouso K , Aoki N , Iwai T , Takashima T , et al . Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res 2012; 42: 376-384.
doi: 10.1111/j.1872-034X.2011.00936.x |
| [6] |
Smajerova M , Petrasova H , Little J , Ovesna P , Andrasina T , Valek V , et al . Contrast-enhanced ultrasonography in the evaluation of incidental focal liver lesions: a cost-effectiveness analysis. World J Gastroenterol 2016; 22: 8605-8614.
doi: 10.3748/wjg.v22.i38.8605 |
| [7] |
Streb JW , Tchelepi H , Malhi H , Deurdulian C , Grant EG . Retrospective analysis of contrast-enhanced ultrasonography effectiveness in reducing time to diagnosis and imaging-related expenditures at a single large United States county hospital. Ultrasound Q 2019; 35: 99-102.
doi: 10.1097/RUQ.0000000000000375 |
| [8] |
Mandai M , Koda M , Matono T , Nagahara T , Sugihara T , Ueki M , et al . Assessment of hepatocellular carcinoma by contrast-enhanced ultrasound with perfluorobutane microbubbles: comparison with dynamic CT. Br J Radiol 2011; 84: 499-507.
doi: 10.1259/bjr/38682601 |
| [9] |
Ding J , Long L , Zhang X , Chen C , Zhou H , Zhou Y , et al . Contrast-enhanced ultrasound LI-RADS 2017: comparison with CT/MRI LI-RADS. Eur Radiol 2021; 31: 847-854.
doi: 10.1007/s00330-020-07159-z |
| [10] |
Huang Z , Zhou P , Li S , Li K . MR versus CEUS LI-RADS for distinguishing hepatocellular carcinoma from other hepatic malignancies in high-risk patients. Ultrasound Med Biol 2021; 47: 1244-1252.
doi: 10.1016/j.ultrasmedbio.2021.01.020 |
| [11] | Hu J , Burrowes DP , Caine BA , Gibson N , Bhayana D , Medellin A , et al. Nodules identified on surveillance ultrasound for HCC: CEUS or MRI as the initial test? J Ultrasound Med 2023; 42:1181-1190. |
| [12] |
Burak KW , Douglas L , Congly SE . Comparing magnetic resonance imaging and Contrast-enhanced ultrasound (CEUS) for the characterization of nodules found on hepatocellular carcinoma surveillance: CEUS is our clear choice. J Ultrasound Med 2023; 42: 1175-1180.
doi: 10.1002/jum.16200 |
| [13] | American College of Radiology. LI-RADS® CEUS v2017 |American College of Radiology. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/CEUS-LI-RADS-2017-Core.pdf. |
| [14] |
Wilson SR , Lyshchik A , Piscaglia F , Cosgrove D , Jang HJ , Sirlin C , et al . CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018; 43: 127-142.
doi: 10.1007/s00261-017-1250-0 |
| [15] |
Hai Y , Savsani E , Chong W , Eisenbrey J , Lyshchik A . Meta-analysis and systematic review of contrast-enhanced ultrasound in evaluating the treatment response after locoregional therapy of hepatocellular carcinoma. Abdom Radiol (NY) 2021; 46: 5162-5179.
doi: 10.1007/s00261-021-03248-9 |
| [16] | Zhang S , Yue M , Shu R , Cheng H , Hu P . Recent advances in the management of hepatocellular carcinoma. J BUON 2016; 21: 307-311 |
| [17] |
Ganesan P , Kulik LM . Hepatocellular carcinoma: new developments. Clin Liver Dis 2023; 27: 85-102.
doi: 10.1016/j.cld.2022.08.004 |
| [18] |
Cho Y , Choi JW , Kwon H , Kim KY , Lee BC , Chu HH , et al . Transarterial chemoembolization for hepatocellular carcinoma: 2023 expert consensus-based practical recommendations of the Korean liver cancer association. Korean J Radiol 2023; 24: 606-625.
doi: 10.3348/kjr.2023.0385 |
| [19] |
European Association for the Study of the Liver. EASL clinical practice guidelines on the management of hepatocellular carcinoma. J Hepatol 2025; 82: 315-374.
doi: 10.1016/j.jhep.2024.08.028 |
| [20] |
Song JE , Kim DY . Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol 2017; 9: 808-814.
doi: 10.4254/wjh.v9.i18.808 |
| [21] |
Gaba RC , Lokken RP , Hickey RM , Lipnik AJ , Lewandowski RJ , Salem R , et al . Quality improvement guidelines for transarterial chemoembolization and embolization of hepatic malignancy. Journal of Vascular and Interventional Radiology 2017; 28: 1210-1223.
doi: 10.1016/j.jvir.2017.04.025 |
| [22] |
Dietrich CF , Lorentzen T , Appelbaum L , Buscarini E , Cantisani V , Correas JM , et al . EFSUMB guidelines on interventional ultrasound (INVUS), Part III-abdominal treatment procedures (short version). Ultraschall Med 2016; 37: 27-45.
doi: 10.1055/s-0035-1553965 |
| [23] |
Martin RCG 2nd , Woeste M , Egger ME , Scoggins CR , McMasters KM , Philips P . Patient selection and outcomes of laparoscopic microwave ablation of hepatocellular carcinoma. Cancers (Basel) 2023; 15: 1965.
doi: 10.3390/cancers15071965 |
| [24] |
Savsani E , Shaw CM , Forsberg F , Wessner CE , Lyshchik A , O'Kane P , et al . Contrast-enhanced US evaluation of hepatocellular carcinoma response to chemoembolization: a prospective multicenter trial. Radiology 2023; 309: e230727.
doi: 10.1148/radiol.230727 |
| [25] |
Cerrito L , Ainora ME , Cuccia G , Galasso L , Mignini I , Esposto G , et al . Dynamic contrast-enhanced ultrasound in the prediction of advanced hepatocellular carcinoma response to systemic and locoregional therapies. Cancers (Basel) 2024; 16: 551.
doi: 10.3390/cancers16030551 |
| [26] |
Eisenbrey JR , Gabriel H , Savsani E , Lyshchik A . Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies. Abdom Radiol (NY) 2021; 46: 3579-3595.
doi: 10.1007/s00261-021-03059-y |
| [27] |
Lyshchik A , Fetzer DT , Kono Y , Wilson SR , Dietrich CF , Clevert DA , et al . Liver imaging reporting and data system contrast-enhanced US nonradiation treatment response assessment version 2024. Radiology 2024; 311: e232369.
doi: 10.1148/radiol.232369 |
| [28] |
Kuon Yeng Escalante CM , Siu Xiao T , Nagaraj RU , Savsani E , Mohammed A , Li J , et al . Evaluation of the contrast-enhanced ultrasound nonradiation treatment response assessment LI-RADS v2024 using data from a multi-center transarterial chemoembolization study. Acad Radiol 2024; 31: 5078-5086.
doi: 10.1016/j.acra.2024.06.005 |
| [29] |
Cao J , Dong Y , Xu X , Zhang Q , Wang W , Möller K , et al . LI-RADS CEUS nonradiation TRA version 2024: application on HCC patients treated with ablation treatment. Ultrasound Med Biol 2025; 51: 1308-1315.
doi: 10.1016/j.ultrasmedbio.2025.04.018 |
| [30] |
Llovet JM , Real MI , Montaña X , Planas R , Coll S , Aponte J , et al . Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359: 1734-1739.
doi: 10.1016/S0140-6736(02)08649-X |
| [31] |
Lo CM , Ngan H , Tso WK , Liu CL , Lam CM , Poon RT , et al . Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002; 35: 1164-1171.
doi: 10.1053/jhep.2002.33156 |
| [32] |
Llovet JM , Bruix J . Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37: 429-442.
doi: 10.1053/jhep.2003.50047 |
| [33] |
Lammer J , Malagari K , Vogl T , Pilleul F , Denys A , Watkinson A , et al . Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010; 33: 41-52.
doi: 10.1007/s00270-009-9711-7 |
| [34] | Razi M , Jianping G , Xu H , Ahmed MJ . Conventional versus drug-eluting bead transarterial chemoembolization: a better option for treatment of unresectable hepatocellular carcinoma. J Interv Med 2020; 4: 11-14 |
| [35] |
Kloeckner R , Weinmann A , Prinz F , Santos DPD , Ruckes C , Duebr C , et al . Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015; 15: 465.
doi: 10.1186/s12885-015-1480-x |
| [36] | Guiu B , Deschamps F , Aho S , Munck F , Dromain C , Boige V , et al . Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol 2012; 56: 609-617 |
| [37] |
Monier A , Guiu B , Duran R , Aho S , Bize P , Deltenre P , et al . Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol 2017; 27: 1431-1439.
doi: 10.1007/s00330-016-4488-y |
| [38] |
Sacco R , Bargellini I , Bertini M , Bozzi E , Romano A , Petruzzi P , et al . Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2011; 22: 1545-1552.
doi: 10.1016/j.jvir.2011.07.002 |
| [39] |
Facciorusso A , Di Maso M , Muscatiello N . Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis 2016; 48: 571-577.
doi: 10.1016/j.dld.2016.02.005 |
| [40] |
Cucchetti A , Trevisani F , Cappelli A , Mosconi C , Renzulli M , Pinna AD , et al . Cost-effectiveness of doxorubicin-eluting beads versus conventional trans-arterial chemo-embolization for hepatocellular carcinoma. Dig Liver Dis 2016; 48: 798-805.
doi: 10.1016/j.dld.2016.03.031 |
| [41] |
Roth GS , Benhamou M , Teyssier Y , Seigneurin A , Abousalihac M , Sengel C , et al . Comparison of trans-arterial chemoembolization and bland embolization for the treatment of hepatocellular carcinoma: a propensity score analysis. Cancers (Basel) 2021; 13: 812.
doi: 10.3390/cancers13040812 |
| [42] |
Tsochatzis EA , Fatourou E , O'Beirne J , Meyer T , Burroughs AK . Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol 2014; 20: 3069-3077.
doi: 10.3748/wjg.v20.i12.3069 |
| [43] |
Xie ZB , Ma L , Wang XB , Bai T , Ye JZ , Zhong JH , et al . Transarterial embolization with or without chemotherapy for advanced hepatocellular carcinoma: a systematic review. Tumour Biol 2014; 35: 8451-8459.
doi: 10.1007/s13277-014-2340-z |
| [44] |
Facciorusso A , Bellanti F , Villani R , Salvatore V , Muscatiello N , Piscaglia F , et al . Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: a meta-analysis of randomized trials. United European Gastroenterol J 2017; 5: 511-518.
doi: 10.1177/2050640616673516 |
| [45] |
Cathomas M , Mueller F , Mertineit N , Baumgartner I , Candinas D , Berzigotti A , et al . Comparison of transarterial bland embolization and drug-eluting beads transarterial chemoembolization for very early and early hepatocellular carcinoma not amenable for surgery or ablation: a single center retrospective data analysis. J Gastrointest Oncol 2023; 14: 2167-2177.
doi: 10.21037/jgo-23-261 |
| [46] |
Hidaka T , Anai H , Sakaguchi H , Sueyoshi S , Tanaka T , Yamamoto K , et al . Efficacy of combined bland embolization and chemoembolization for huge (≥ 10 cm) hepatocellular carcinoma. Minim Invasive Ther Allied Technol 2021; 30: 221-228.
doi: 10.1080/13645706.2020.1725580 |
| [47] |
Ding H , Kudo M , Onda H , Suetomi Y , Minami Y , Chung H , et al . Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology 2001; 221: 721-730.
doi: 10.1148/radiol.2213010358 |
| [48] | Takizawa K , Numata K , Morimoto M , Kondo M , Nozaki A , Moriya S , et al. Use of contrast-enhanced ultrasonography with a perflubutane-based contrast agent performed one day after transarterial chemoembolization for the early assessment of residual viable hepatocellular carcinoma. Eur J Radiol 2013; 82:1471-1480. |
| [49] |
Watanabe Y , Ogawa M , Kumagawa M , Hirayama M , Miura T , Matsumoto N , et al . Utility of contrast-enhanced ultrasound for early therapeutic evaluation of hepatocellular carcinoma after transcatheter arterial chemoembolization. J Ultrasound Med 2020; 39: 431-440.
doi: 10.1002/jum.15118 |
| [50] |
Shaw CM , Eisenbrey JR , Lyshchik A , O'Kane PL , Merton DA , Machado P , et al . Contrast-enhanced ultrasound evaluation of residual blood flow to hepatocellular carcinoma after treatment with transarterial chemoembolization using drug-eluting beads: a prospective study. J Ultrasound Med 2015; 34: 859-867.
doi: 10.7863/ultra.34.5.859 |
| [51] |
Shiozawa K , Matsui T , Murakami T , Watanabe M , Maetani I . Predicting therapeutic efficacy of transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma using contrast-enhanced ultrasound. Diagnostics (Basel) 2021; 11: 291.
doi: 10.3390/diagnostics11020291 |
| [52] |
Forner A , Reig M , Bruix J . Hepatocellular carcinoma. Lancet 2018; 391: 1301-1314.
doi: 10.1016/S0140-6736(18)30010-2 |
| [53] |
Head HW , Dodd GD 3rd . Thermal ablation for hepatocellular carcinoma. Gastroenterology 2004; 127: S167-178.
doi: 10.1053/j.gastro.2004.09.031 |
| [54] |
Kim YS , Lim HK , Rhim H , Lee MW . Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2014; 28: 897-908.
doi: 10.1016/j.bpg.2014.08.011 |
| [55] |
Cucchetti A , Piscaglia F , Cescon M , Colecchia A , Ercolani G , Bolondi L , et al . Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013; 59: 300-307.
doi: 10.1016/j.jhep.2013.04.009 |
| [56] |
Faccia M , Garcovich M , Ainora ME , Riccardi L , Pompili M , Gasbarrini A , et al . Contrast-enhanced ultrasound for monitoring treatment response in different stages of hepatocellular carcinoma. Cancers (Basel) 2022; 14: 481.
doi: 10.3390/cancers14030481 |
| [57] |
Bale R , Schullian P , Eberle G , Putzer D , Zoller H , Schneeberger S , et al . Stereotactic radiofrequency ablation of hepatocellular carcinoma: a histopathological study in explanted livers. Hepatology 2019; 70: 840-850.
doi: 10.1002/hep.30406 |
| [58] |
Izzo F , Granata V , Grassi R , Fusco R , Palaia R , Delrio P , et al . Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist 2019; 24: e990-e1005.
doi: 10.1634/theoncologist.2018-0337 |
| [59] |
Lu MD , Xu HX , Xie XY , Yin XY , Chen JW , Kuang M , et al . Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 2005; 40: 1054-1060.
doi: 10.1007/s00535-005-1671-3 |
| [60] |
Fu Y , Zhu Q , Zhao X , Lu J , Wang W . Efficacy and safety of ultrasound-guided percutaneous microwave ablation for hepatocellular carcinoma at specific anatomic sites of the liver: a systematic review and meta-analysis. BMC Gastroenterol 2025; 25: 505.
doi: 10.1186/s12876-025-04081-w |
| [61] |
Yu Q , Liu C , Navuluri R , Ahmed O . Percutaneous microwave ablation versus radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Abdom Radiol (NY) 2021; 46: 4467-4475.
doi: 10.1007/s00261-021-03080-1 |
| [62] | Tan W , Deng Q , Lin S , Wang Y , Xu G . Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 2019; 36: 264-272 |
| [63] |
Potretzke TA , Ziemlewicz TJ , Hinshaw JL , Lubner MG , Wells SA , Brace CL , et al . Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center. J Vasc Interv Radiol 2016; 27: 631-638.
doi: 10.1016/j.jvir.2016.01.136 |
| [64] |
Sun B , Zhang W , Chen L , Sun T , Ren Y , Zhu L , et al . The safety and efficacy of percutaneous ethanol injection in the treatment of tumor thrombus in advanced hepatocellular carcinoma with portal vein tumor thrombus. Abdom Radiol (NY) 2022; 47: 858-868.
doi: 10.1007/s00261-021-03349-5 |
| [65] |
Ebara M , Okabe S , Kita K , Sugiura N , Fukuda H , Yoshikawa M , et al . Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol 2005; 43: 458-564.
doi: 10.1016/j.jhep.2005.03.033 |
| [66] | Pompili M , Nicolardi E , Abbate V , Miele L , Riccardi L , Covino M , et al . Ethanol injection is highly effective for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol 2011; 17: 3126-3132 |
| [67] |
Salvaggio G , Campisi A , Lo Greco V , Cannella I , Meloni MF , Caruso G . Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging 2010; 35: 447-453.
doi: 10.1007/s00261-009-9551-6 |
| [68] |
Wiggermann P , Zuber-Jerger I , Zausig Y , Loss M , Scherer MN , Schreyer AG , et al . Contrast-enhanced ultrasound improves real-time imaging of ablation region during radiofrequency ablation: preliminary results. Clin Hemorheol Microcirc 2011; 49: 43-54.
doi: 10.3233/CH-2011-1456 |
| [69] |
Gallotti A , D'Onofrio M , Ruzzenente A , Martone E , De Robertis R , Guglielmi A , et al . Contrast-enhanced ultrasonography (CEUS) immediately after percutaneous ablation of hepatocellular carcinoma. Radiol Med 2009; 114: 1094-1105.
doi: 10.1007/s11547-009-0436-0 |
| [70] |
Ainora ME , Iezzi R , Ponziani FR , Garcovich M , Di Stasio E , Riccardi L , et al . Contrast-enhanced ultrasound in the short-term evaluation of hepatocellular carcinoma after locoregional treatment. Dig Dis 2020; 38: 522-533.
doi: 10.1159/000506455 |
| [71] |
Bartolotta TV , Taibbi A , Midiri M , De Maria M . Hepatocellular cancer response to radiofrequency tumor ablation: contrast-enhanced ultrasound. Abdom Imaging 2008; 33: 501-511.
doi: 10.1007/s00261-007-9294-1 |
| [72] | Pregler B , Beyer LP , Wiesinger I , Nießen C , Jung EM , Stroszczynski C , et al . Microwave ablation of large HCC lesions: added value of CEUS examinations for ablation success control. Clin Hemorheol Microcirc 2016; 64: 483-490 |
| [73] |
Qu P , Yu X , Liang P , Cheng Z , Han Z , Liu F , et al . Contrast-enhanced ultrasound in the characterization of hepatocellular carcinomas treated by ablation: comparison with contrast-enhanced magnetic resonance imaging. Ultrasound Med Biol 2013; 39: 1571-1579.
doi: 10.1016/j.ultrasmedbio.2013.03.030 |
| [74] |
Lu MD , Yu XL , Li AH , Jiang TA , Chen MH , Zhao BZ , et al . Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 2007; 33: 1736-1749.
doi: 10.1016/j.ultrasmedbio.2007.05.004 |
| [75] |
Du J , Li HL , Zhai B , Chang S , Li FH . Radiofrequency ablation for hepatocellular carcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in guiding and assessing early therapeutic response and short-term follow-up results. Ultrasound Med Biol 2015; 41: 2400-2411.
doi: 10.1016/j.ultrasmedbio.2015.05.004 |
| [76] |
Catalano O , Izzo F , Vallone P , Sandomenico F , Albino V , Nunziata A , et al . Integrating contrast-enhanced sonography in the follow-up algorithm of hepatocellular carcinoma treated with radiofrequency ablation: single cancer center experience. Acta Radiol 2015; 56: 133-142.
doi: 10.1177/0284185114521108 |
| [77] |
Kim TK , Khalili K , Jang HJ . Local ablation therapy with contrast-enhanced ultrasonography for hepatocellular carcinoma: a practical review. Ultrasonography 2015; 34: 235-245.
doi: 10.14366/usg.15018 |
| [78] |
Dong J , Hu X , Li L , Jing FY , Chen JJ , Chen J . Diagnostic performance of contrast-enhanced ultrasonography for predicting therapeutic response to radiofrequency ablation in patients with hepatocellular carcinoma: a meta-analysis. Discov Oncol 2025; 16: 1237.
doi: 10.1007/s12672-025-03008-x |
| [79] |
Zheng SG , Xu HX , Lu MD , Xie XY , Xu ZF , Liu GJ , et al . Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J Gastroenterol 2013; 19: 855-865.
doi: 10.3748/wjg.v19.i6.855 |
| [80] | Long H , Zhou X , Zhang X , Ye J , Huang T , Cong L , et al . 3D fusion is superior to 2D point-to-point contrast-enhanced US to evaluate the ablative margin after RFA for hepatocellular carcinoma. Eur Radiol 2024; 34: 1247-1257 |
| [81] |
Lu YB , Huang YN , Weng YC , Chiang TY , Fang TK , Chen WT , et al . Contrast-enhanced ultrasonography guidance avoids US-CT/MR fusion error for percutaneous radiofrequency ablation of hepatocellular carcinoma. BMC Med Imaging 2024; 24: 323.
doi: 10.1186/s12880-024-01508-w |
| [82] |
Ye J , Huang G , Zhang X , Xu M , Zhou X , Lin M , et al . Three-dimensional contrast-enhanced ultrasound fusion imaging predicts local tumor progression by evaluating ablative margin of radiofrequency ablation for hepatocellular carcinoma: a preliminary report. Int J Hyperthermia 2019; 36: 55-64.
doi: 10.1080/02656736.2018.1530460 |
| [83] |
Wang Y , Jing X , Ding J . Clinical value of dynamic 3-dimensional contrast-enhanced ultrasound imaging for the assessment of hepatocellular carcinoma ablation. Clin Imaging 2016; 40: 402-406.
doi: 10.1016/j.clinimag.2015.11.022 |
| [84] |
Delaney LJ , Tantawi M , Wessner CE , Machado P , Forsberg F , Lyshchik A , et al . Predicting long-term hepatocellular carcinoma response to transarterial radioembolization using contrast-enhanced ultrasound: initial experiences. Ultrasound Med Biol 2021; 47: 2523-2531.
doi: 10.1016/j.ultrasmedbio.2021.05.006 |
| [1] | Yu Xiao jie, Song Zheng lai, Chang Xue yong, Yu Jie, Liang Ping. Artificial Intelligence in Ultrasound Diagnosis of Liver Nodules: A Comprehensive Review of B-Mode and Contrast-enhanced Applications [J]. Advanced Ultrasound in Diagnosis and Therapy, 2025, 9(4): 326-346. |
| [2] | Guan Xin, Hu Xinyuan, Han Hong, Zhang Dezhi, Xu Huixiong. The Evolving Application of Ultrasound in the Precision Management of Small Hepatocellular Carcinoma [J]. Advanced Ultrasound in Diagnosis and Therapy, 2025, 9(4): 375-387. |
| [3] | Yue Tian, MB, Yanan Zhao, MD, Yiran Huang, MB. The Diagnostic Value of Real-time Shear Wave Ultrasound Elastography in the Differentiation of Hepatic Hemangioma and Hepatocellular Carcinoma [J]. Advanced Ultrasound in Diagnosis and Therapy, 2024, 8(3): 124-129. |
| [4] | Osama Mahmoud, BS, Ajay Makkena, BS, Corinne E. Wessner, MS, MBA, RDMS, Ji-Bin Liu, MD, John R. Eisenbrey, PhD, Andrej Lyshchik, MD, PhD. Contrast-Enhanced Ultrasound LI-RADS: A Pictorial Review [J]. Advanced Ultrasound in Diagnosis and Therapy, 2023, 7(4): 321-332. |
| [5] | Esika Savsani, Mohamed Tantawi, MD, Corinne E. Wessner, MBA, RDMS, RVT, Philip Lee, MD, Andrej Lyshchik, MD, PhD, Kevin Anton, MD, PhD, Colette M. Shaw, MD, Ji-Bin Liu, MD, John R. Eisenbrey, PhD. Contrast-enhanced Ultrasound Assessment of Treatment Response in a Patient with Multifocal Hepatocellular Carcinoma Treated with Transarterial Chemo and Radioembolization [J]. Advanced Ultrasound in Diagnosis and Therapy, 2021, 5(3): 254-257. |
| [6] | Mengna He, MD, PHD, Lu Zhu, MD, Tianan Jiang, MD, PHD. Findings of Fat Containing Hepatocellular Carcinoma on Contrast-enhanced Ultrasound with Sonazoid: A Case Report [J]. Advanced Ultrasound in Diagnosis and Therapy, 2021, 5(2): 98-101. |
| [7] | Yan Zhou, Jianmin Ding, Fengmei Wang, Zhengyi Qin, Yandong Wang, Hongyu Zhou, Xiang Jing. The Effects of Liver Function Damage after Thermal Ablation on the Prognosis of HCC Patients and Its Prediction [J]. Advanced Ultrasound in Diagnosis and Therapy, 2021, 5(2): 80-86. |
| [8] | Mengna He, MD, PhD, Lei Xu, MD, Tian’an Jiang, MD, PhD. Time-intensity Curve Analysis of Hepatocellular Carcinoma using Two Contrast-enhanced Ultrasound Methods: Contrast Pulse Sequencing and Contrast Harmonic Imaging [J]. Advanced Ultrasound in Diagnosis and Therapy, 2020, 4(3): 217-222. |
| [9] | Li Ma, MD, Wenzhao Liang, MD, Yupeng Zhu, MD, Yingqiao Zhu, MD, Dezhi Zhang, MD. Differences in CEUS and CE-MRI Appearance of HCC: A Case Report [J]. Advanced Ultrasound in Diagnosis and Therapy, 2019, 3(4): 197-199. |
| [10] | Meng Li, MD, Zhiyan Li, MD, Yuejuan Gao, MD, Jiangke Tian, MD, Min Chen, MD, Jinghui Dong, MD. Safety and Efficacy of Percutaneous Radiofrequency Ablation Combined with Percutaneous Ethanol Injection for Hepatocellular Carcinoma in High-risk Locations [J]. Advanced Ultrasound in Diagnosis and Therapy, 2018, 2(1): 1-7. |
| [11] | Xianghong Luo, MD, Qian Zhang, MD, Qing Yan, MD, Jufang Wang, MD, Qingqing Dong, MD, Zhaojun Li, MD. Diagnosis with Echocardiography for Rare Cases of Anomalous Left Main Coronary Artery: Two Case Reports and Important Lessons Learned [J]. Advanced Ultrasound in Diagnosis and Therapy, 2017, 1(1): 15-18. |
| [12] | Jianmin Ding, MD, Yan Zhou, MD, Yandong Wang, MD, Hongyu Zhou, MD, Xiang Jing, MD. Clinical Value of Contrast-enhanced Ultrasound in Differential Diagnosis of Early Hepatocellular Carcinoma and Dysplastic Nodules [J]. Advanced Ultrasound in Diagnosis and Therapy, 2017, 1(1): 10-14. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Share: WeChat
Copyright ©2018 Advanced Ultrasound in Diagnosis and Therapy
|
 Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
Advanced Ultrasound in Diagnosis and Therapy (AUDT)
is licensed under a Creative Commons Attribution 4.0 International License.
